The E3 ubiquitin ligase seven in absentia homolog 1 may be a potential new therapeutic target for Parkinson's disease
- PMID: 26487857
- PMCID: PMC4590242
- DOI: 10.4103/1673-5374.162763
The E3 ubiquitin ligase seven in absentia homolog 1 may be a potential new therapeutic target for Parkinson's disease
Abstract
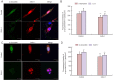
In this study, we investigated the effect of an antibody against E3 ubiquitin ligase seven in absentia homolog 1 (SIAH-1) in PC12 cells. 1-Methyl-4-phenylpyridinium (MPP(+)) treatment increased α-synuclein, E1 and SIAH-1 protein levels in PC12 cells, and it reduced cell viability; however, there was no significant change in light chain 3 expression. Treatment with an SIAH-1 antibody decreased mRNA expression levels of α-synuclein, light chain 3 and SIAH-1, but increased E1 mRNA expression. It also increased cell viability. Combined treatment with MPP(+) and rapamycin reduced SIAH-1 and α-synuclein levels. Treatment with SIAH-1 antibody alone diminished α-synuclein immunoreactivity in PC12 cells, and reduced the colocalization of α-synuclein and light chain 3. These findings suggest that the SIAH-1 antibody reduces the monoubiquitination and aggregation of α-synuclein, promoting its degradation by the ubiquitin-proteasome pathway. Consequently, SIAH-1 may be a potential new therapeutic target for Parkinson's disease.
Keywords: 1-methyl-4-phenylpyridinium; E3 ubiquitin ligase seven in absentia homolog 1; Parkinson's disease; autophagy; nerve regeneration; neural regeneration; neurodegeneration; rapamycin; ubiquitin-proteasome system.
Conflict of interest statement
Figures



References
-
- Cai ZL, Shi JJ, Yang YP. MPP + impairs autophagic clearance of alpha-synuclein by impairing the activity of dynein. Neuroreport. 2009;20:569–573. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
