Automatic sequential fluid handling with multilayer microfluidic sample isolated pumping
- PMID: 26487904
- PMCID: PMC4592428
- DOI: 10.1063/1.4932303
Automatic sequential fluid handling with multilayer microfluidic sample isolated pumping
Abstract
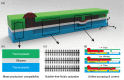
To sequentially handle fluids is of great significance in quantitative biology, analytical chemistry, and bioassays. However, the technological options are limited when building such microfluidic sequential processing systems, and one of the encountered challenges is the need for reliable, efficient, and mass-production available microfluidic pumping methods. Herein, we present a bubble-free and pumping-control unified liquid handling method that is compatible with large-scale manufacture, termed multilayer microfluidic sample isolated pumping (mμSIP). The core part of the mμSIP is the selective permeable membrane that isolates the fluidic layer from the pneumatic layer. The air diffusion from the fluidic channel network into the degassing pneumatic channel network leads to fluidic channel pressure variation, which further results in consistent bubble-free liquid pumping into the channels and the dead-end chambers. We characterize the mμSIP by comparing the fluidic actuation processes with different parameters and a flow rate range of 0.013 μl/s to 0.097 μl/s is observed in the experiments. As the proof of concept, we demonstrate an automatic sequential fluid handling system aiming at digital assays and immunoassays, which further proves the unified pumping-control and suggests that the mμSIP is suitable for functional microfluidic assays with minimal operations. We believe that the mμSIP technology and demonstrated automatic sequential fluid handling system would enrich the microfluidic toolbox and benefit further inventions.
Figures





Similar articles
-
Elastic membrane enabled inward pumping for liquid manipulation on a centrifugal microfluidic platform.Biomicrofluidics. 2022 May 18;16(3):034105. doi: 10.1063/5.0089112. eCollection 2022 May. Biomicrofluidics. 2022. PMID: 35607410 Free PMC article.
-
Bubble pump: scalable strategy for in-plane liquid routing.Lab Chip. 2015 Jul 7;15(13):2842-53. doi: 10.1039/c5lc00326a. Epub 2015 May 28. Lab Chip. 2015. PMID: 26016773
-
Triggering vacuum capillaries for pneumatic pumping and metering liquids in point-of-care immunoassays.Lab Chip. 2008 Jul;8(7):1216-9. doi: 10.1039/b801878b. Epub 2008 May 16. Lab Chip. 2008. PMID: 18584101
-
Centrifugal microfluidic platforms: advanced unit operations and applications.Chem Soc Rev. 2015 Oct 7;44(17):6187-229. doi: 10.1039/c4cs00371c. Epub 2015 Jun 2. Chem Soc Rev. 2015. PMID: 26035697 Review.
-
Photo-actuation of liquids for light-driven microfluidics: state of the art and perspectives.Lab Chip. 2012 Oct 7;12(19):3637-53. doi: 10.1039/c2lc40596b. Lab Chip. 2012. PMID: 22864577 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
