Abscisic Acid Stimulates Glucagon-Like Peptide-1 Secretion from L-Cells and Its Oral Administration Increases Plasma Glucagon-Like Peptide-1 Levels in Rats
- PMID: 26488296
- PMCID: PMC4619318
- DOI: 10.1371/journal.pone.0140588
Abscisic Acid Stimulates Glucagon-Like Peptide-1 Secretion from L-Cells and Its Oral Administration Increases Plasma Glucagon-Like Peptide-1 Levels in Rats
Abstract
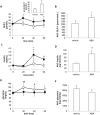
In recent years, Abscisic Acid (ABA) has been demonstrated to be involved in the regulation of glucose homeostasis in mammals as an endogenous hormone, by stimulating both insulin release and peripheral glucose uptake. In addition, ABA is released by glucose- or GLP-1-stimulated β-pancreatic cells. Here we investigated whether ABA can stimulate GLP-1 release. The human enteroendocrine L cell line hNCI-H716 was used to explore whether ABA stimulates in vitro GLP-1 secretion and/or transcription. ABA induced GLP-1 release in hNCI-H716 cells, through a cAMP/PKA-dependent mechanism. ABA also enhanced GLP-1 transcription. In addition, oral administration of ABA significantly increased plasma GLP-1 and insulin levels in rats. In conclusion, ABA can stimulate GLP-1 release: this result and the previous observation that GLP-1 stimulates ABA release from β -cells, suggest a positive feed-back mechanism between ABA and GLP-1, regulating glucose homeostasis. Type 2 diabetes treatments targeting the GLP-1 axis by either inhibiting its rapid clearance by dipeptidyl-peptidase IV or using GLP-1 mimetics are currently used. Moreover, the development of treatments aimed at stimulating GLP-1 release from L cells has been considered as an alternative approach. Accordingly, our finding that ABA increases GLP-1 release in vitro and in vivo may suggest ABA and/or ABA analogs as potential anti-diabetic treatments.
Conflict of interest statement
Figures


Similar articles
-
Biophysical and pharmacological properties of glucagon-like peptide-1 in rats under isoflurane anesthesia.Anesth Analg. 2012 Jul;115(1):62-9. doi: 10.1213/ANE.0b013e318253cbf0. Epub 2012 Apr 13. Anesth Analg. 2012. PMID: 22504208
-
Ezetimibe stimulates intestinal glucagon-like peptide 1 secretion via the MEK/ERK pathway rather than dipeptidyl peptidase 4 inhibition.Metabolism. 2015 May;64(5):633-41. doi: 10.1016/j.metabol.2015.02.001. Epub 2015 Feb 8. Metabolism. 2015. PMID: 25704082
-
A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like Peptide-1 and glucose-dependent insulinotropic Peptide release.Endocrinology. 2008 May;149(5):2038-47. doi: 10.1210/en.2007-0966. Epub 2008 Jan 17. Endocrinology. 2008. PMID: 18202141
-
Glucagon-like peptide 1-potentiated insulin secretion and proliferation of pancreatic β-cells.J Diabetes. 2014 Sep;6(5):394-402. doi: 10.1111/1753-0407.12161. Epub 2014 May 22. J Diabetes. 2014. PMID: 24725840 Review.
-
Potential new approaches to modifying intestinal GLP-1 secretion in patients with type 2 diabetes mellitus: focus on bile acid sequestrants.Clin Drug Investig. 2012 Jan 1;32(1):1-14. doi: 10.2165/11595370-000000000-00000. Clin Drug Investig. 2012. PMID: 21958333 Review.
Cited by
-
Recent Progress in the Diagnosis and Management of Type 2 Diabetes Mellitus in the Era of COVID-19 and Single Cell Multi-Omics Technologies.Life (Basel). 2022 Aug 8;12(8):1205. doi: 10.3390/life12081205. Life (Basel). 2022. PMID: 36013384 Free PMC article. Review.
-
Abscisic Acid: A Novel Nutraceutical for Glycemic Control.Front Nutr. 2017 Jun 13;4:24. doi: 10.3389/fnut.2017.00024. eCollection 2017. Front Nutr. 2017. PMID: 28660193 Free PMC article. Review.
-
Phytohormone abscisic acid ameliorates neuropathic pain via regulating LANCL2 protein abundance and glial activation at the spinal cord.Mol Pain. 2022 Apr;18:17448069221107781. doi: 10.1177/17448069221107781. Mol Pain. 2022. PMID: 35647699 Free PMC article.
-
Abscisic acid ameliorates d-galactose -induced aging in mice by modulating AMPK-SIRT1-p53 pathway and intestinal flora.Heliyon. 2024 Mar 16;10(6):e28283. doi: 10.1016/j.heliyon.2024.e28283. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38524603 Free PMC article.
-
Is There A Role for Abscisic Acid, A Proven Anti-Inflammatory Agent, in the Treatment of Ischemic Retinopathies?Antioxidants (Basel). 2019 Apr 17;8(4):104. doi: 10.3390/antiox8040104. Antioxidants (Basel). 2019. PMID: 30999583 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

