Heterodimerization of Two Pathological Mutants Enhances the Activity of Human Phosphomannomutase2
- PMID: 26488408
- PMCID: PMC4619449
- DOI: 10.1371/journal.pone.0139882
Heterodimerization of Two Pathological Mutants Enhances the Activity of Human Phosphomannomutase2
Abstract
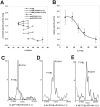
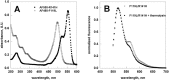
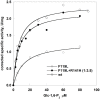
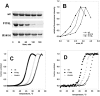
The most frequent disorder of glycosylation is due to mutations in the gene encoding phosphomannomutase2 (PMM2-CDG). For this disease, which is autosomal and recessive, there is no cure at present. Most patients are composite heterozygous and carry one allele encoding an inactive mutant, R141H, and one encoding a hypomorphic mutant. Phosphomannomutase2 is a dimer. We reproduced composite heterozygosity in vitro by mixing R141H either with the wild type protein or the most common hypomorphic mutant F119L and compared the quaternary structure, the activity and the stability of the heterodimeric enzymes. We demonstrated that the activity of R141H/F119L heterodimers in vitro, which reproduces the protein found in patients, has the same activity of wild type/R141H, which reproduces the protein found in healthy carriers. On the other hand the stability of R141H/F119L appears to be reduced both in vitro and in vivo. These findings suggest that a therapy designed to enhance protein stability such as those based on pharmacological chaperones or modulation of proteostasis could be beneficial for PMM2-CDG patients carrying R141H/F119L genotype as well as for other genotypes where protein stability rather than specific activity is affected by mutations.
Conflict of interest statement
Figures





References
-
- Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372(3):774–97. . - PubMed
-
- HGMD. HGMD: http://www.hgmd.cf.ac.uk/ac/index.php. 2011. 1954227.
-
- Schollen E, Kjaergaard S, Legius E, Schwartz M, Matthijs G. Lack of Hardy-Weinberg equilibrium for the most prevalent PMM2 mutation in CDG-Ia (congenital disorders of glycosylation type Ia). Eur J Hum Genet. 2000;8(5):367–71. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

