Characterizing Cellular Biophysical Responses to Stress by Relating Density, Deformability, and Size
- PMID: 26488647
- PMCID: PMC4624161
- DOI: 10.1016/j.bpj.2015.08.038
Characterizing Cellular Biophysical Responses to Stress by Relating Density, Deformability, and Size
Abstract
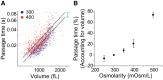
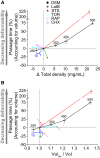
Cellular physical properties are important indicators of specific cell states. Although changes in individual biophysical parameters, such as cell size, density, and deformability, during cellular processes have been investigated in great detail, relatively little is known about how they are related. Here, we use a suspended microchannel resonator (SMR) to measure single-cell density, volume, and passage time through a narrow constriction of populations of cells subjected to a variety of environmental stresses. Osmotic stress significantly affects density and volume, as previously shown. In contrast to density and volume, the effect of an osmotic challenge on passage time is relatively small. Deformability, as determined by comparing passage times for cells with similar volume, exhibits a strong dependence on osmolarity, indicating that passage time alone does not always provide a meaningful proxy for deformability. Finally, we find that protein synthesis inhibition, cell-cycle arrest, protein kinase inhibition, and cytoskeletal disruption result in unexpected relationships among deformability, density, and volume. Taken together, our results suggest that by measuring multiple biophysical parameters, one can detect unique characteristics that more specifically reflect cellular behaviors.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Cross S.E., Jin Y.-S., Gimzewski J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007;2:780–783. - PubMed
-
- Tse H.T.K., Gossett D.R., Di Carlo D. Quantitative diagnosis of malignant pleural effusions by single-cell mechanophenotyping. Sci. Transl. Med. 2013;5:212ra163. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

