1,3-Oxazin-6-one Derivatives and Bohemamine-Type Pyrrolizidine Alkaloids from a Marine-Derived Streptomyces spinoverrucosus
- PMID: 26489038
- PMCID: PMC5435457
- DOI: 10.1021/acs.jnatprod.5b00604
1,3-Oxazin-6-one Derivatives and Bohemamine-Type Pyrrolizidine Alkaloids from a Marine-Derived Streptomyces spinoverrucosus
Abstract
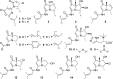
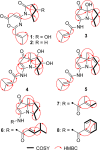
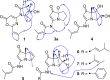
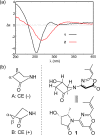
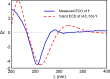
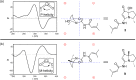
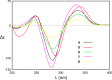
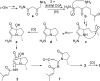
Two new 1,3-oxazin-6-one derivatives (1 and 2) and six new bohemamine-type pyrrolizidine alkaloids (3-8) were isolated from the marine-derived Streptomyces spinoverrucosus strain SNB-048. Their structures including the absolute configurations were fully elucidated on the basis of spectroscopic analysis, ECD spectra, quantum chemical calculations, and chemical methods. Compounds 1 and 2 possess a γ-lactam moiety and a 1,3-oxazin-6-one system.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
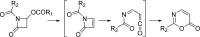

Similar articles
-
Spithioneines A and B, Two New Bohemamine Derivatives Possessing Ergothioneine Moiety from a Marine-Derived Streptomyces spinoverrucosus.Org Lett. 2015 Jun 19;17(12):3046-9. doi: 10.1021/acs.orglett.5b01328. Epub 2015 May 29. Org Lett. 2015. PMID: 26024315 Free PMC article.
-
Studies Related to the Proposed Biotransformation of Bohemamine D into the Co-occurring Marine Natural Product Spinoxazine B.J Nat Prod. 2025 Apr 25;88(4):1004-1011. doi: 10.1021/acs.jnatprod.5c00109. Epub 2025 Mar 31. J Nat Prod. 2025. PMID: 40162671
-
Bohemamines from a marine-derived Streptomyces sp.J Nat Prod. 2006 Nov;69(11):1626-8. doi: 10.1021/np0602721. J Nat Prod. 2006. PMID: 17125235
-
Pyrrolizidine alkaloids.Nat Prod Rep. 1992 Aug;9(4):313-21. doi: 10.1039/np9920900313. Nat Prod Rep. 1992. PMID: 1522978 Review. No abstract available.
-
Pyrrolizidine alkaloids.Nat Prod Rep. 1990 Oct;7(5):377-86. doi: 10.1039/np9900700377. Nat Prod Rep. 1990. PMID: 2077451 Review. No abstract available.
Cited by
-
Aminoacyl chain translocation catalysed by a type II thioesterase domain in an unusual non-ribosomal peptide synthetase.Nat Commun. 2022 Jan 10;13(1):62. doi: 10.1038/s41467-021-27512-0. Nat Commun. 2022. PMID: 35013184 Free PMC article.
-
Synthesis of legonmycins A and B, C(7a)-hydroxylated bacterial pyrrolizidines.Beilstein J Org Chem. 2021 Feb 2;17:334-342. doi: 10.3762/bjoc.17.31. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 33828615 Free PMC article.
-
Genomic scanning enabling discovery of a new antibacterial bicyclic carbamate-containing alkaloid.Synth Syst Biotechnol. 2021 Jan 20;6(1):12-19. doi: 10.1016/j.synbio.2021.01.002. eCollection 2021 Mar. Synth Syst Biotechnol. 2021. PMID: 33553705 Free PMC article.
-
Dehydroamino acid residues in bioactive natural products.Nat Prod Rep. 2024 Feb 21;41(2):273-297. doi: 10.1039/d3np00041a. Nat Prod Rep. 2024. PMID: 37942836 Free PMC article. Review.
-
Expanding the chemical diversity of an endophytic fungus Bulgaria inquinans, an ascomycete associated with mistletoe, through an OSMAC approach.RSC Adv. 2019 Aug 13;9(43):25119-25132. doi: 10.1039/c9ra03678d. eCollection 2019 Aug 8. RSC Adv. 2019. PMID: 35528664 Free PMC article.
References
-
- Grote R.; Zeeck A.; Stümpfel J.; Zähner H. Liebigs Ann. Chem. 1990, 1990, 525–530. 10.1002/jlac.1990199001100. - DOI
-
- Hu J. F.; Wunderlich D.; Thiericke R.; Dahse H. M.; Grabley S.; Feng X. Z.; Sattler I. J. Antibiot. 2003, 56, 747–754. 10.7164/antibiotics.56.747. - DOI - PubMed
- Snider B. B.; Duvall J. R.; Sattler I. Tetrahedron Lett. 2004, 45, 6725–6727. 10.1016/j.tetlet.2004.07.055. - DOI
- Duvall J. R.; Wu F.; Snider B. B. J. Org. Chem. 2006, 71, 8579–8590. 10.1021/jo061650+. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

