Zebrafish Models for Human Acute Organophosphorus Poisoning
- PMID: 26489395
- PMCID: PMC4614985
- DOI: 10.1038/srep15591
Zebrafish Models for Human Acute Organophosphorus Poisoning
Erratum in
-
Corrigendum: Zebrafish Models for Human Acute Organophosphorus Poisoning.Sci Rep. 2016 Jan 7;6:17244. doi: 10.1038/srep17244. Sci Rep. 2016. PMID: 26738741 Free PMC article. No abstract available.
Abstract
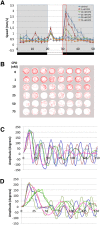
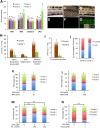
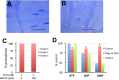
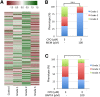
Terrorist use of organophosphorus-based nerve agents and toxic industrial chemicals against civilian populations constitutes a real threat, as demonstrated by the terrorist attacks in Japan in the 1990 s or, even more recently, in the Syrian civil war. Thus, development of more effective countermeasures against acute organophosphorus poisoning is urgently needed. Here, we have generated and validated zebrafish models for mild, moderate and severe acute organophosphorus poisoning by exposing zebrafish larvae to different concentrations of the prototypic organophosphorus compound chlorpyrifos-oxon. Our results show that zebrafish models mimic most of the pathophysiological mechanisms behind this toxidrome in humans, including acetylcholinesterase inhibition, N-methyl-D-aspartate receptor activation, and calcium dysregulation as well as inflammatory and immune responses. The suitability of the zebrafish larvae to in vivo high-throughput screenings of small molecule libraries makes these models a valuable tool for identifying new drugs for multifunctional drug therapy against acute organophosphorus poisoning.
Figures



References
-
- Peña Llopis S. & Pena L. Antioxidants as Potentially Safe Antidotes for Organophosphorus Poisoning. Current enzyme inhibition 1, 147–156 (2005).
-
- Shih T. Ä. & McDonough J. H. Neurochemical Mechanisms in Soman-induced Seizures. J Appl Toxicol 17, 255–264 (1997). - PubMed
-
- Tryphonas L. & Clement J. G. Histomorphogenesis of soman-induced encephalocardiomyopathy in Sprague-Dawley rats. Toxicol Pathol 23, 393–409 (1995). - PubMed
-
- Weissman B. A. & Raveh L. Therapy against organophosphate poisoning: the importance of anticholinergic drugs with antiglutamatergic properties. Toxicol Appl Pharmacol 232, 351–358 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

