Prolyl Isomerase Pin1 Regulates Axon Guidance by Stabilizing CRMP2A Selectively in Distal Axons
- PMID: 26489457
- PMCID: PMC4662601
- DOI: 10.1016/j.celrep.2015.09.026
Prolyl Isomerase Pin1 Regulates Axon Guidance by Stabilizing CRMP2A Selectively in Distal Axons
Abstract
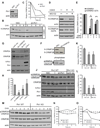
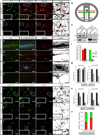
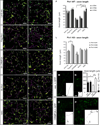
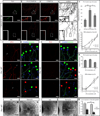
Axon guidance relies on precise translation of extracellular signal gradients into local changes in cytoskeletal dynamics, but the molecular mechanisms regulating dose-dependent responses of growth cones are still poorly understood. Here, we show that during embryonic development in growing axons, a low level of Semaphorin3A stimulation is buffered by the prolyl isomerase Pin1. We demonstrate that Pin1 stabilizes CDK5-phosphorylated CRMP2A, the major isoform of CRMP2 in distal axons. Consequently, Pin1 knockdown or knockout reduces CRMP2A levels specifically in distal axons and inhibits axon growth, which can be fully rescued by Pin1 or CRMP2A expression. Moreover, Pin1 knockdown or knockout increases sensitivity to Sema3A-induced growth cone collapse in vitro and in vivo, leading to developmental abnormalities in axon guidance. These results identify an important isoform-specific function and regulation of CRMP2A in controlling axon growth and uncover Pin1-catalyzed prolyl isomerization as a regulatory mechanism in axon guidance.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Arimura N, Inagaki N, Chihara K, Menager C, Nakamura N, Amano M, Iwamatsu A, Goshima Y, Kaibuchi K. Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J Biol Chem. 2000;275:23973–23980. - PubMed
-
- Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

