Evolutionary landscape of the Mycobacterium tuberculosis complex from the viewpoint of PhoPR: implications for virulence regulation and application to vaccine development
- PMID: 26489860
- PMCID: PMC4620462
- DOI: 10.1128/mBio.01289-15
Evolutionary landscape of the Mycobacterium tuberculosis complex from the viewpoint of PhoPR: implications for virulence regulation and application to vaccine development
Abstract
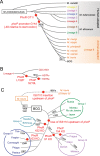
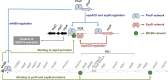
Different members of the Mycobacterium genus have evolved to cause tuberculosis in diverse human populations and in a variety of animal species. Our cumulative knowledge of mycobacterial genomes indicates that mutations in the PhoPR two-component virulence system were acquired not only during the natural evolution of mycobacterial species but also during in vitro subculture, which has given rise to the attenuated reference strain H37Ra or to different daughter strains of Mycobacterium bovis BCG. PhoPR is a well-known regulator of pathogenic phenotypes, including secretion of the virulence factor ESAT-6, biosynthesis of acyltrehalose-based lipids, and modulation of antigen export, in members of the Mycobacterium tuberculosis complex (MTBC). Evolutionarily conserved polymorphisms in PhoPR from Mycobacterium africanum, M. bovis, or M. tuberculosis H37Ra result in loss of functional phenotypes. Interestingly, some members of the MTBC have acquired compensatory mutations to counteract these polymorphisms and, probably, to maintain their pathogenic potential. Some of these compensatory mutations include the insertion of the IS6110 element upstream from phoPR in a particular M. bovis strain that is able to transmit between humans or polymorphisms in M. africanum and M. bovis that affect the regulatory region of the espACD operon, allowing PhoPR-independent ESAT-6 secretion. This review highlights the increasing knowledge of the significance of PhoPR in the evolution of the MTBC and its potential application in the construction of new attenuated vaccines based on phoPR inactivation. In this context, the live attenuated vaccine MTBVAC, based on a phoP fadD26 deletion mutant of M. tuberculosis, is the first vaccine of this kind to successfully enter into clinical development, representing a historic milestone in the field of human vaccinology.
Copyright © 2015 Broset et al.
Figures


References
-
- Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, Eiglmeier K, Garnier T, Gutierrez C, Hewinson G, Kremer K, Parsons LM, Pym AS, Samper S, van Soolingen D, Cole ST. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A 99:3684–3689. doi:10.1073/pnas.052548299. - DOI - PMC - PubMed
-
- Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, Parkhill J, Malla B, Berg S, Thwaites G, Yeboah-Manu D, Bothamley G, Mei J, Wei L, Bentley S, Harris SR, Niemann S, Diel R, Aseffa A, Gao Q, Young D, Gagneux S. 2013. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet 45:1176–1182. doi:10.1038/ng.2744. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
