MKS1 regulates ciliary INPP5E levels in Joubert syndrome
- PMID: 26490104
- PMCID: PMC5060087
- DOI: 10.1136/jmedgenet-2015-103250
MKS1 regulates ciliary INPP5E levels in Joubert syndrome
Abstract
Background: Joubert syndrome (JS) is a recessive ciliopathy characterised by a distinctive brain malformation 'the molar tooth sign'. Mutations in >27 genes cause JS, and mutations in 12 of these genes also cause Meckel-Gruber syndrome (MKS). The goals of this work are to describe the clinical features of MKS1-related JS and determine whether disease causing MKS1 mutations affect cellular phenotypes such as cilium number, length and protein content as potential mechanisms underlying JS.
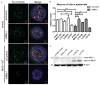
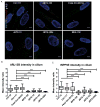
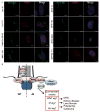
Methods: We measured cilium number, length and protein content (ARL13B and INPP5E) by immunofluorescence in fibroblasts from individuals with MKS1-related JS and in a three-dimensional (3D) spheroid rescue assay to test the effects of disease-related MKS1 mutations.
Results: We report MKS1 mutations (eight of them previously unreported) in nine individuals with JS. A minority of the individuals with MKS1-related JS have MKS features. In contrast to the truncating mutations associated with MKS, all of the individuals with MKS1-related JS carry ≥ 1 non-truncating mutation. Fibroblasts from individuals with MKS1-related JS make normal or fewer cilia than control fibroblasts, their cilia are more variable in length than controls, and show decreased ciliary ARL13B and INPP5E. Additionally, MKS1 mutant alleles have similar effects in 3D spheroids.
Conclusions: MKS1 functions in the transition zone at the base of the cilium to regulate ciliary INPP5E content, through an ARL13B-dependent mechanism. Mutations in INPP5E also cause JS, so our findings in patient fibroblasts support the notion that loss of INPP5E function, due to either mutation or mislocalisation, is a key mechanism underlying JS, downstream of MKS1 and ARL13B.
Keywords: Cell biology; Genetics.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Tobin JLP, Beales PLBMDF. The nonmotile ciliopathies. Genetics in Medicine. 2009;11(6):386–402. - PubMed
-
- Parisi MA, Doherty D, Chance PF, Glass IA. Joubert syndrome (and related disorders) (OMIM 213300) Eur J Hum Genet. 2007;15(5):511–21. - PubMed
-
- Joubert M, Eisenring JJ, Robb JP, Andermann F. Familial agenesis of the cerebellar vermis. A syndrome of episodic hyperpnea, abnormal eye movements, ataxia, and retardation. Neurology. 1969;19(9):813–25. - PubMed
-
- Steinlin M, Schmid M, Landau K, Boltshauser E. Follow-Up in Children with Joubert Syndrome. Neuropediatr. 1997;28:204–11. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- KL2-RR025015/RR/NCRR NIH HHS/United States
- U54 HD083091/HD/NICHD NIH HHS/United States
- K24HD046712/HD/NICHD NIH HHS/United States
- DK068306/DK/NIDDK NIH HHS/United States
- P30 HD002274/HD/NICHD NIH HHS/United States
- R01 DK068306/DK/NIDDK NIH HHS/United States
- RC4-DK090917/DK/NIDDK NIH HHS/United States
- Howard Hughes Medical Institute/United States
- DK090917/DK/NIDDK NIH HHS/United States
- R01NS064077/NS/NINDS NIH HHS/United States
- KL2 TR002317/TR/NCATS NIH HHS/United States
- P30HD002274/HD/NICHD NIH HHS/United States
- KL2 RR025015/RR/NCRR NIH HHS/United States
- MR/K011154/1/Medical Research Council/United Kingdom
- RC4 DK090917/DK/NIDDK NIH HHS/United States
- K23 NS045832/NS/NINDS NIH HHS/United States
- K24 HD046712/HD/NICHD NIH HHS/United States
- R01 NS064077/NS/NINDS NIH HHS/United States
- K23NS45832/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
