Downregulation of programmed cell death 10 is associated with tumor cell proliferation, hyperangiogenesis and peritumoral edema in human glioblastoma
- PMID: 26490252
- PMCID: PMC4618952
- DOI: 10.1186/s12885-015-1709-8
Downregulation of programmed cell death 10 is associated with tumor cell proliferation, hyperangiogenesis and peritumoral edema in human glioblastoma
Abstract
Background: Neovascularization and peritumoral edema are hallmarks of glioblastoma (GBM). Programmed cell death 10 (PDCD10) plays a pivotal role in regulating apoptosis, neoangiogenesis and vessel permeability and is implicated in certain tumor signaling pathways. However, little is known about PDCD10 in GBM. We aimed to investigate the expression pattern of PDCD10 and to identify the association of its expression with some molecular and clinical parameters in human GBM.
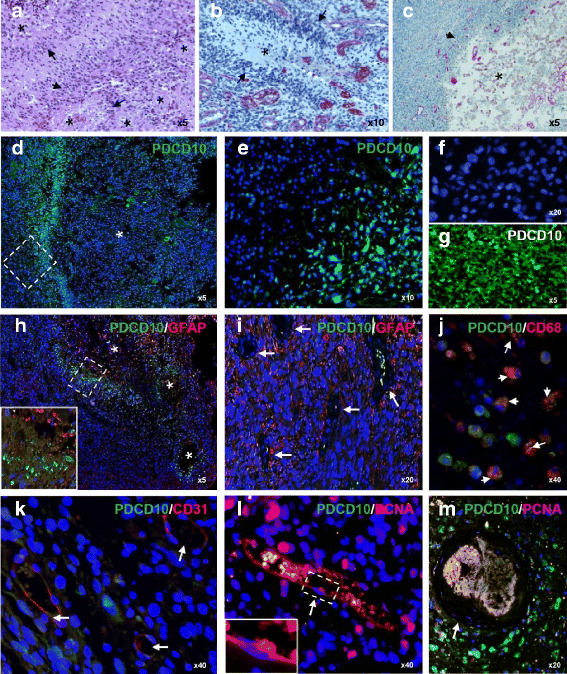
Methods: mRNA and protein expression of PDCD10 were examined respectively by real-time RT-PCR and Western blotting in GBM (n = 27), astrocytoma grade II (n = 13) and control (n = 11). The protein level of p-Akt and GFAP was detected by Western blot. Double-imunofluorecent staining was performed to reveal the cellular expression profile of PDCD10. Brain edema and microvascular density (MVD) were respectively analyzed based on pre-operative MRI and after laminin immnostaining. MGMT promoter methylation was detected by methylation specific PCR.
Results: mRNA and protein levels of PDCD10 were significantly downregulated in GBM, concomitantly accompanied by the activation of Akt. PDCD10 immunoreactivity was absent in proliferating tumor cells, endothelial cells and GFAP-positive cells, but exclusively present in the hypoxic pseudopalisading cells which underwent apoptosis. Moreover, loss of PDCD10 was associated with a higher MVD and a more severe peritumoral edema but not with MGMT promoter methylation in GBM.
Conclusion: We report for the first time that PDCD10 expression is downregulated in GBM, which is associated with the activation of Akt signaling protein. PDCD10 is potentially implicated in tumor proliferation and apoptosis, hyperangiogenesis and peritumoral edema in GBM.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

