Systemic Tolerance Mediated by Melanoma Brain Tumors Is Reversible by Radiotherapy and Vaccination
- PMID: 26490306
- PMCID: PMC4825863
- DOI: 10.1158/1078-0432.CCR-15-1516
Systemic Tolerance Mediated by Melanoma Brain Tumors Is Reversible by Radiotherapy and Vaccination
Abstract
Purpose: Immune responses to antigens originating in the central nervous system (CNS) are generally attenuated, as collateral damage can have devastating consequences. The significance of this finding for the efficacy of tumor-targeted immunotherapies is largely unknown.
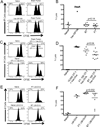
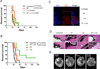
Experimental design: The B16 murine melanoma model was used to compare cytotoxic responses against established tumors in the CNS and in the periphery. Cytokine analysis of tissues from brain tumor-bearing mice detected elevated TGFβ secretion from microglia and in the serum and TGFβ signaling blockade reversed tolerance of tumor antigen-directed CD8 T cells. In addition, a treatment regimen using focal radiation therapy and recombinant Listeria monocytogenes was evaluated for immunologic activity and efficacy in this model.
Results: CNS melanomas were more tolerogenic than equivalently progressed tumors outside the CNS as antigen-specific CD8 T cells were deleted and exhibited impaired cytotoxicity. Tumor-bearing mice had elevated serum levels of TGFβ; however, blocking TGFβ signaling with a small-molecule inhibitor or a monoclonal antibody did not improve survival. Conversely, tumor antigen-specific vaccination in combination with focal radiation therapy reversed tolerance and improved survival. This treatment regimen was associated with increased polyfunctionality of CD8 T cells, elevated T effector to T regulatory cell ratios, and decreased TGFβ secretion from microglia.
Conclusions: These data suggest that CNS tumors may impair systemic antitumor immunity and consequently accelerate cancer progression locally as well as outside the CNS, whereas antitumor immunity may be restored by combining vaccination with radiation therapy. These findings are hypothesis-generating and warrant further study in contemporary melanoma models as well as human trials.
©2015 American Association for Cancer Research.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

