Analyzing pathogenic (double-stranded (ds) DNA-specific) plasma cells via immunofluorescence microscopy
- PMID: 26490351
- PMCID: PMC4618946
- DOI: 10.1186/s13075-015-0811-2
Analyzing pathogenic (double-stranded (ds) DNA-specific) plasma cells via immunofluorescence microscopy
Abstract
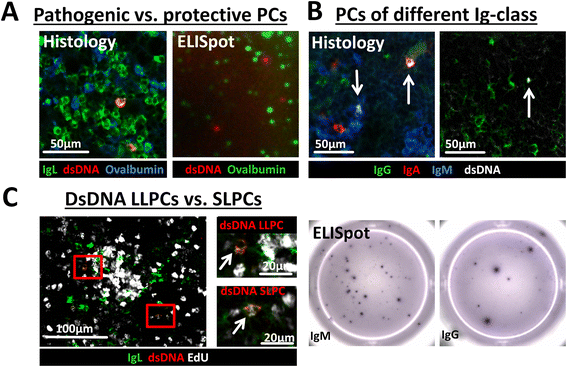
Introduction: While protective plasma cells (PCs) are an important part of the individual's immune defense, autoreactive plasma cells such as dsDNA-specific plasma cells contribute to the pathogenesis of autoimmune diseases like systemic lupus erythematosus (SLE). However, the research on dsDNA-specific plasma cells was restricted to the ELISpot technique, with its limitations, as no other attempt for identification of dsDNA-reactive plasma cells had been successful.
Methods: With improved fluorochrome labeling of dsDNA, removal of DNA aggregates, and enhanced blocking of unspecific binding, we were able to specifically detect dsDNA-reactive plasma cells by immunofluorescence microscopy.
Results: Via this novel technique we were able to distinguish short-lived (SLPCs) and long-lived (LLPCs) autoreactive plasma cells, discriminate dsDNA-specific plasma cells according to their immunoglobulin class (IgG, IgM, and IgA) and investigate autoreactive (dsDNA) and vaccine-induced ovalbumin (Ova) plasma cells in parallel.
Conclusions: The detection of autoreactive dsDNA-specific plasma cells via immunofluorescence microscopy allows specific studies on pathogenic and protective plasma cell subsets and their niches, detailed evaluation of therapeutic treatments and therefore offers new possibilities for basic and clinical research.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

