Peroxidase-catalysed interfacial adhesion of aquatic caddisworm silk
- PMID: 26490632
- PMCID: PMC4685843
- DOI: 10.1098/rsif.2015.0710
Peroxidase-catalysed interfacial adhesion of aquatic caddisworm silk
Abstract
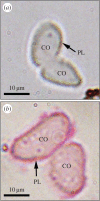
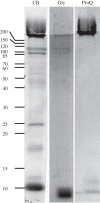
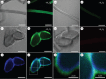
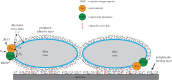
Casemaker caddisfly (Hesperophylax occidentalis) larvae use adhesive silk fibres to construct protective shelters under water. The silk comprises a distinct peripheral coating on a viscoelastic fibre core. Caddisworm silk peroxinectin (csPxt), a haem-peroxidase, was shown to be glycosylated by lectin affinity chromatography and tandem mass spectrometry. Using high-resolution H2O2 and peroxidase-dependent silver ion reduction and nanoparticle deposition, imaged by electron microscopy, csPxt activity was shown to be localized in the peripheral layer of drawn silk fibres. CsPxt catalyses dityrosine cross-linking within the adhesive peripheral layer post-draw, initiated perhaps by H2O2 generated by a silk gland-specific superoxide dismutase 3 (csSOD3) from environmental reactive oxygen species present in natural water. CsSOD3 was also shown to be a glycoprotein and is likely localized in the peripheral layer. Using a synthetic fluorescent phenolic copolymer and confocal microscopy, it was shown that csPxt catalyses oxidative cross-linking to external polyphenolic compounds capable of diffusive interpenetration into the fuzzy peripheral coating, including humic acid, a natural surface-active polyphenol. The results provide evidence of enzyme-mediated covalent cross-linking of a natural bioadhesive to polyphenol conditioned interfaces as a mechanism of permanent adhesion underwater.
Keywords: bioadhesive; caddisworms; dityrosine cross-linking; peroxinectin; silk.
© 2015 The Author(s).
Figures





Similar articles
-
Peroxinectin catalyzed dityrosine crosslinking in the adhesive underwater silk of a casemaker caddisfly larvae, Hysperophylax occidentalis.Insect Biochem Mol Biol. 2014 Nov;54:69-79. doi: 10.1016/j.ibmb.2014.08.009. Epub 2014 Sep 16. Insect Biochem Mol Biol. 2014. PMID: 25220661
-
Aquatic caddisworm silk is solidified by environmental metal ions during the natural fiber-spinning process.FASEB J. 2019 Jan;33(1):572-583. doi: 10.1096/fj.201801029R. Epub 2018 Jul 9. FASEB J. 2019. PMID: 29985645
-
Exploring the underwater silken architectures of caddisworms: comparative silkomics across two caddisfly suborders.Philos Trans R Soc Lond B Biol Sci. 2019 Oct 28;374(1784):20190206. doi: 10.1098/rstb.2019.0206. Epub 2019 Sep 9. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31495307 Free PMC article.
-
Connecting caddisworm silk structure and mechanical properties: combined infrared spectroscopy and mechanical analysis.Open Biol. 2016 Jun;6(6):160067. doi: 10.1098/rsob.160067. Open Biol. 2016. PMID: 27278649 Free PMC article.
-
Unraveling the genetics of underwater caddisfly silk.Trends Genet. 2025 Jun;41(6):537-546. doi: 10.1016/j.tig.2025.01.004. Epub 2025 Jan 31. Trends Genet. 2025. PMID: 39893090 Review.
Cited by
-
Sequence basis of Barnacle Cement Nanostructure is Defined by Proteins with Silk Homology.Sci Rep. 2016 Nov 8;6:36219. doi: 10.1038/srep36219. Sci Rep. 2016. PMID: 27824121 Free PMC article.
-
Reversible Oxidative Modifications in Myoglobin and Functional Implications.Antioxidants (Basel). 2020 Jun 24;9(6):549. doi: 10.3390/antiox9060549. Antioxidants (Basel). 2020. PMID: 32599765 Free PMC article.
-
High Performance Marine and Terrestrial Bioadhesives and the Biomedical Applications They Have Inspired.Molecules. 2022 Dec 16;27(24):8982. doi: 10.3390/molecules27248982. Molecules. 2022. PMID: 36558114 Free PMC article. Review.
-
In Silico Analysis of Pacific Oyster (Crassostrea gigas) Transcriptome over Developmental Stages Reveals Candidate Genes for Larval Settlement.Int J Mol Sci. 2019 Jan 8;20(1):197. doi: 10.3390/ijms20010197. Int J Mol Sci. 2019. PMID: 30625986 Free PMC article.
-
Draft Genome Assemblies and Annotations of Agrypnia vestita Walker, and Hesperophylax magnus Banks Reveal Substantial Repetitive Element Expansion in Tube Case-Making Caddisflies (Insecta: Trichoptera).Genome Biol Evol. 2021 Mar 1;13(3):evab013. doi: 10.1093/gbe/evab013. Genome Biol Evol. 2021. PMID: 33501983 Free PMC article.
References
-
- Sehnal F. 2008. Prospects of the practical use of silk sericins. Entomol. Res. 38, S1–S8. (10.1111/j.1748-5967.2008.00168.x) - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

