Peroxidase-catalysed interfacial adhesion of aquatic caddisworm silk
- PMID: 26490632
- PMCID: PMC4685843
- DOI: 10.1098/rsif.2015.0710
Peroxidase-catalysed interfacial adhesion of aquatic caddisworm silk
Abstract
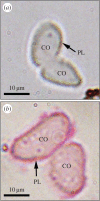
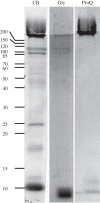
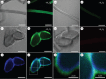
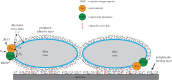
Casemaker caddisfly (Hesperophylax occidentalis) larvae use adhesive silk fibres to construct protective shelters under water. The silk comprises a distinct peripheral coating on a viscoelastic fibre core. Caddisworm silk peroxinectin (csPxt), a haem-peroxidase, was shown to be glycosylated by lectin affinity chromatography and tandem mass spectrometry. Using high-resolution H2O2 and peroxidase-dependent silver ion reduction and nanoparticle deposition, imaged by electron microscopy, csPxt activity was shown to be localized in the peripheral layer of drawn silk fibres. CsPxt catalyses dityrosine cross-linking within the adhesive peripheral layer post-draw, initiated perhaps by H2O2 generated by a silk gland-specific superoxide dismutase 3 (csSOD3) from environmental reactive oxygen species present in natural water. CsSOD3 was also shown to be a glycoprotein and is likely localized in the peripheral layer. Using a synthetic fluorescent phenolic copolymer and confocal microscopy, it was shown that csPxt catalyses oxidative cross-linking to external polyphenolic compounds capable of diffusive interpenetration into the fuzzy peripheral coating, including humic acid, a natural surface-active polyphenol. The results provide evidence of enzyme-mediated covalent cross-linking of a natural bioadhesive to polyphenol conditioned interfaces as a mechanism of permanent adhesion underwater.
Keywords: bioadhesive; caddisworms; dityrosine cross-linking; peroxinectin; silk.
© 2015 The Author(s).
Figures





References
-
- Sehnal F. 2008. Prospects of the practical use of silk sericins. Entomol. Res. 38, S1–S8. (10.1111/j.1748-5967.2008.00168.x) - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
