F4+ ETEC infection and oral immunization with F4 fimbriae elicits an IL-17-dominated immune response
- PMID: 26490738
- PMCID: PMC4618862
- DOI: 10.1186/s13567-015-0264-2
F4+ ETEC infection and oral immunization with F4 fimbriae elicits an IL-17-dominated immune response
Abstract
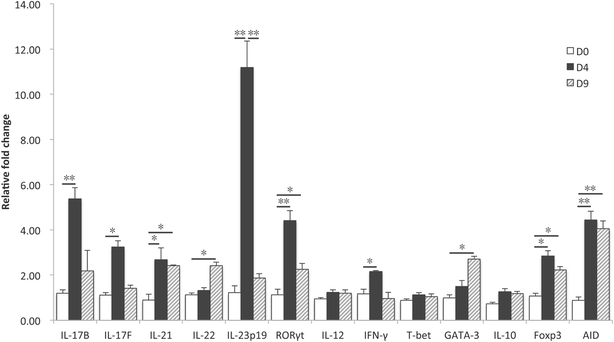
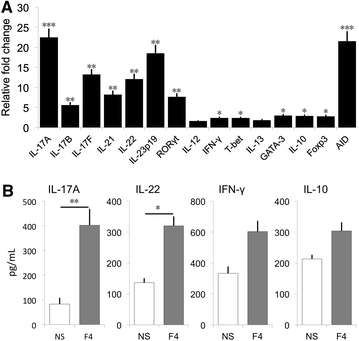
Enterotoxigenic Escherichia coli (ETEC) are an important cause of post-weaning diarrhea (PWD) in piglets. Porcine-specific ETEC strains possess different fimbrial subtypes of which F4 fimbriae are the most frequently associated with ETEC-induced diarrhea in piglets. These F4 fimbriae are potent oral immunogens that induce protective F4-specific IgA antibody secreting cells at intestinal tissues. Recently, T-helper 17 (Th17) cells have been implicated in the protection of the host against extracellular pathogens. However, it remains unknown if Th17 effector responses are needed to clear ETEC infections. In the present study, we aimed to elucidate if ETEC elicits a Th17 response in piglets and if F4 fimbriae trigger a similar response. F4(+) ETEC infection upregulated IL-17A, IL-17F, IL-21 and IL-23p19, but not IL-12 and IFN-γ mRNA expression in the systemic and mucosal immune system. Similarly, oral immunization with F4 fimbriae triggered a Th17 signature evidenced by an upregulated mRNA expression of IL-17F, RORγt, IL-23p19 and IL-21 in the peripheral blood mononuclear cells (PBMCs). Intriguingly, IL-17A mRNA levels were unaltered. To further evaluate this difference between systemic and mucosal immune responses, we assayed the cytokine mRNA profile of F4 fimbriae stimulated PBMCs. F4 fimbriae induced IL-17A, IL-17F, IL-22 and IL-23p19, but downregulated IL-17B mRNA expression. Altogether, these data indicate a Th17 dominated response upon oral immunization with F4 fimbriae and F4(+) ETEC infection. Our work also highlights that IL-17B and IL-17F participate in the immune response to protect the host against F4(+) ETEC infection and could aid in the design of future ETEC vaccines.
Figures





Similar articles
-
Heterologous prime-boost immunization of two-component vaccine candidate PWDVax protected pigs against F18 enterotoxigenic Escherichia coli post-weaning diarrhea.Infect Immun. 2025 Apr 8;93(4):e0040624. doi: 10.1128/iai.00406-24. Epub 2025 Mar 12. Infect Immun. 2025. PMID: 40071919 Free PMC article.
-
The effect of enterotoxigenic Escherichia coli F4ab,ac on early-weaned piglets: a gene expression study.Vet Immunol Immunopathol. 2013 Mar 15;152(1-2):87-92. doi: 10.1016/j.vetimm.2012.09.027. Epub 2012 Sep 26. Vet Immunol Immunopathol. 2013. PMID: 23078902
-
Targeting of Escherichia coli F4 fimbriae to Fcgamma receptors enhances the maturation of porcine dendritic cells.Vet Immunol Immunopathol. 2010 Jun 15;135(3-4):188-98. doi: 10.1016/j.vetimm.2009.11.013. Epub 2009 Dec 3. Vet Immunol Immunopathol. 2010. PMID: 20022123
-
Receptor for the F4 fimbriae of enterotoxigenic Escherichia coli (ETEC).Appl Microbiol Biotechnol. 2015 Jun;99(12):4953-9. doi: 10.1007/s00253-015-6643-9. Epub 2015 May 13. Appl Microbiol Biotechnol. 2015. PMID: 25967654 Review.
-
ETEC vaccination in pigs.Vet Immunol Immunopathol. 2013 Mar 15;152(1-2):37-42. doi: 10.1016/j.vetimm.2012.09.024. Epub 2012 Sep 26. Vet Immunol Immunopathol. 2013. PMID: 23068270 Review.
Cited by
-
RNA-seq reveals a novel porcine lncRNA MPHOSPH9-OT1 induces CXCL8/IL-8 expression in ETEC infected IPEC-J2 cells.Front Cell Infect Microbiol. 2022 Aug 25;12:996841. doi: 10.3389/fcimb.2022.996841. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36093177 Free PMC article.
-
Potential immunosuppressive effects of Escherichia coli O157:H7 experimental infection on the bovine host.BMC Genomics. 2016 Dec 21;17(1):1049. doi: 10.1186/s12864-016-3374-y. BMC Genomics. 2016. PMID: 28003017 Free PMC article.
-
The lipopolysaccharide structure affects the detoxifying ability of intestinal alkaline phosphatases.BMC Vet Res. 2024 Aug 10;20(1):358. doi: 10.1186/s12917-024-04208-3. BMC Vet Res. 2024. PMID: 39127648 Free PMC article.
-
Animal Enterotoxigenic Escherichia coli.EcoSal Plus. 2016 Oct;7(1):10.1128/ecosalplus.ESP-0006-2016. doi: 10.1128/ecosalplus.ESP-0006-2016. EcoSal Plus. 2016. PMID: 27735786 Free PMC article. Review.
-
Intestinal and systemic inflammation induced by symptomatic and asymptomatic enterotoxigenic E. coli infection and impact on intestinal colonization and ETEC specific immune responses in an experimental human challenge model.Gut Microbes. 2021 Jan-Dec;13(1):1-13. doi: 10.1080/19490976.2021.1891852. Gut Microbes. 2021. PMID: 33645430 Free PMC article.
References
-
- Francis DH. Enterotoxigenic Escherichia coli infection in pigs and its diagnosis. J Swine Health Prod. 2002;10:171–175.
-
- Wang J, Jiang SW, Chen XH, Liu ZL, Peng J. Prevalence of fimbrial antigen (K88 variants, K99 and 987P) of enterotoxigenic Escherichia coli from neonatal and post-weaning piglets with diarrhea in central China. Asian Aust J Anim Sci. 2006;19:1342–1346. doi: 10.5713/ajas.2006.1342. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

