Branch-Selective Alkene Hydroarylation by Cooperative Destabilization: Iridium-Catalyzed ortho-Alkylation of Acetanilides
- PMID: 26490739
- PMCID: PMC4691333
- DOI: 10.1002/anie.201506581
Branch-Selective Alkene Hydroarylation by Cooperative Destabilization: Iridium-Catalyzed ortho-Alkylation of Acetanilides
Abstract
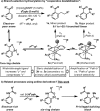
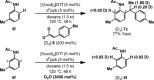
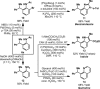
An iridium(I) catalyst system, modified with the wide-bite-angle and electron-deficient bisphosphine d(F) ppb (1,4-bis(di(pentafluorophenyl)phosphino)butane) promotes highly branch-selective hydroarylation reactions between diverse acetanilides and aryl- or alkyl-substituted alkenes. This provides direct and ortho-selective access to synthetically challenging anilines, and addresses long-standing issues associated with related Friedel-Crafts alkylations.
Keywords: acetanilides; branch selectivity; hydroarylation; iridium; phosphine ligands.
© 2015 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures

References
-
- Rappoport Z, editor. The Chemistry of Anilines, Parts 1 and 2. New York: Wiley; 2007.
-
- Wei Z, Faul CFJ. Macromol. Rapid Commun. 2008;29:280.
-
- Lundgren RJ, Stradiotto M. Aldrichimica Acta. 2012;45:59. For a recent review on the Buchwald–Hartwig amination, see.
-
- Rao KS, Wu T-S. Tetrahedron. 2012;68:7735. For a review on the Chan–Lam coupling, see.
-
- Evano G, Blanchard N, Toumi M. Chem. Rev. 2008;108:3054. For a review that encompasses the Ullmann coupling, see. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

