Ryanodine receptor cluster fragmentation and redistribution in persistent atrial fibrillation enhance calcium release
- PMID: 26490742
- PMCID: PMC4648199
- DOI: 10.1093/cvr/cvv231
Ryanodine receptor cluster fragmentation and redistribution in persistent atrial fibrillation enhance calcium release
Abstract
Aims: In atrial fibrillation (AF), abnormalities in Ca(2+) release contribute to arrhythmia generation and contractile dysfunction. We explore whether ryanodine receptor (RyR) cluster ultrastructure is altered and is associated with functional abnormalities in AF.
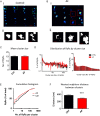
Methods and results: Using high-resolution confocal microscopy (STED), we examined RyR cluster morphology in fixed atrial myocytes from sheep with persistent AF (N = 6) and control (Ctrl; N = 6) animals. RyR clusters on average contained 15 contiguous RyRs; this did not differ between AF and Ctrl. However, the distance between clusters was significantly reduced in AF (288 ± 12 vs. 376 ± 17 nm). When RyR clusters were grouped into Ca(2+) release units (CRUs), i.e. clusters separated by <150 nm, CRUs in AF had more clusters (3.43 ± 0.10 vs. 2.95 ± 0.02 in Ctrl), which were more dispersed. Furthermore, in AF cells, more RyR clusters were found between Z lines. In parallel experiments, Ca(2+) sparks were monitored in live permeabilized myocytes. In AF, myocytes had >50% higher spark frequency with increased spark time to peak (TTP) and duration, and a higher incidence of macrosparks. A computational model of the CRU was used to simulate the morphological alterations observed in AF cells. Increasing cluster fragmentation to the level observed in AF cells caused the observed changes, i.e. higher spark frequency, increased TTP and duration; RyR clusters dispersed between Z-lines increased the occurrence of macrosparks.
Conclusion: In persistent AF, ultrastructural reorganization of RyR clusters within CRUs is associated with overactive Ca(2+) release, increasing the likelihood of propagating Ca(2+) release.
Keywords: Atrial fibrillation; Atrial myocytes; Ryanodine receptor; Sarcoplasmic reticulum; Super-resolution microscopy.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures





References
-
- Camm AJ, Lip GY, De CR, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. Eur Heart J 2012;33:2719–2747. - PubMed
-
- Dobrev D, Carlsson L, Nattel S. Novel molecular targets for atrial fibrillation therapy. Nat Rev Drug Discov 2012;11:275–291. - PubMed
-
- Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res 2010;106:1134–1144. - PubMed
-
- Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012;125:2059–2070. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

