Disrupting Cyclin Dependent Kinase 1 in Spermatocytes Causes Late Meiotic Arrest and Infertility in Mice
- PMID: 26490841
- PMCID: PMC4712696
- DOI: 10.1095/biolreprod.115.134940
Disrupting Cyclin Dependent Kinase 1 in Spermatocytes Causes Late Meiotic Arrest and Infertility in Mice
Abstract
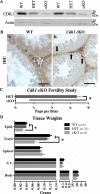
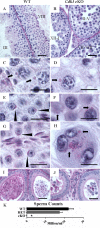
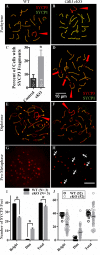
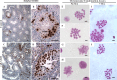
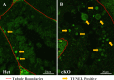
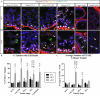
While cyclin dependent kinase 1 (CDK1) has a critical role in controlling resumption of meiosis in oocytes, its role has not been investigated directly in spermatocytes. Unique aspects of male meiosis led us to hypothesize that its role is different in male meiosis than in female meiosis. We generated a conditional knockout (cKO) of the Cdk1 gene in mouse spermatocytes to test this hypothesis. We found that CDK1-null spermatocytes undergo synapsis, chiasmata formation, and desynapsis as is seen in oocytes. Additionally, CDK1-null spermatocytes relocalize SYCP3 to centromeric foci, express H3pSer10, and initiate chromosome condensation. However, CDK1-null spermatocytes fail to form condensed bivalent chromosomes in prophase of meiosis I and instead are arrested at prometaphase. Thus, CDK1 has an essential role in male meiosis that is consistent with what is known about the role of CDK1 in female meiosis, where it is required for formation of condensed bivalent metaphase chromosomes and progression to the first meiotic division. We found that cKO spermatocytes formed fully condensed bivalent chromosomes in the presence of okadaic acid, suggesting that cKO chromosomes are competent to condense, although they do not do so in vivo. Additionally, arrested cKO spermatocytes exhibited irregular cell shape, irregular large nuclei, and large distinctive nucleoli. These cells persist in the seminiferous epithelium through the next seminiferous epithelial cycle with a lack of stage XII checkpoint-associated cell death. This indicates that CDK1 is required upstream of a checkpoint-associated cell death as well as meiotic metaphase progression in mouse spermatocytes.
Keywords: CDK1; cell cycle; chromosome condensation; male meiosis; meiotic arrest; metaphase-promoting factor; okadaic acid; synaptonemal complex.
© 2015 by the Society for the Study of Reproduction, Inc.
Figures
Similar articles
-
Acquisition of competence to condense metaphase I chromosomes during spermatogenesis.Dev Biol. 1999 Jan 1;205(1):49-64. doi: 10.1006/dbio.1998.9101. Dev Biol. 1999. PMID: 9882497
-
Meiotic prophase abnormalities and metaphase cell death in MLH1-deficient mouse spermatocytes: insights into regulation of spermatogenic progress.Dev Biol. 2002 Sep 1;249(1):85-95. doi: 10.1006/dbio.2002.0708. Dev Biol. 2002. PMID: 12217320
-
Loss of TDP-43 in male germ cells causes meiotic failure and impairs fertility in mice.J Biol Chem. 2021 Nov;297(5):101231. doi: 10.1016/j.jbc.2021.101231. Epub 2021 Sep 29. J Biol Chem. 2021. PMID: 34599968 Free PMC article.
-
What are the spermatocyte's requirements for successful meiotic division?J Exp Zool. 1999 Oct 15;285(3):243-50. J Exp Zool. 1999. PMID: 10497323 Review.
-
MEIOSIN directs initiation of meiosis and subsequent meiotic prophase program during spermatogenesis.Genes Genet Syst. 2022 Jun 4;97(1):27-39. doi: 10.1266/ggs.21-00054. Epub 2021 Dec 25. Genes Genet Syst. 2022. PMID: 34955498 Review.
Cited by
-
Divergence Analyses of Sperm DNA Methylomes between Monozygotic Twin AI Bulls.Epigenomes. 2019 Sep 26;3(4):21. doi: 10.3390/epigenomes3040021. Epigenomes. 2019. PMID: 34968253 Free PMC article.
-
Testicular abnormalities in mice with Y chromosome deficiencies.Biol Reprod. 2017 Mar 1;96(3):694-706. doi: 10.1095/biolreprod.116.144006. Biol Reprod. 2017. PMID: 28339606 Free PMC article.
-
The Spo13/Meikin pathway confines the onset of gamete differentiation to meiosis II in yeast.EMBO J. 2022 Feb 15;41(4):e109446. doi: 10.15252/embj.2021109446. Epub 2022 Jan 13. EMBO J. 2022. PMID: 35023198 Free PMC article.
-
PRSS50 is a testis protease responsible for proper sperm tail formation and function.Development. 2021 Apr 15;148(8):dev197558. doi: 10.1242/dev.197558. Epub 2021 Apr 16. Development. 2021. PMID: 33913480 Free PMC article.
-
Proteome Informatics in Tibetan Sheep (Ovis aries) Testes Suggest the Crucial Proteins Related to Development and Functionality.Front Vet Sci. 2022 Jul 15;9:923789. doi: 10.3389/fvets.2022.923789. eCollection 2022. Front Vet Sci. 2022. PMID: 35909681 Free PMC article.
References
-
- Adhikari D, Zheng W, Shen Y, Gorre N, Ning Y, Halet G, Kaldis P, Liu K. Cdk1, but not Cdk2, is the sole Cdk that is essential and sufficient to drive resumption of meiosis in mouse oocytes Hum Mol Genet 2012. 21 2476– 2484 - PubMed
-
- Santamaria D, Barriere C, Cerqueira A, Hunt S, Tardy C, Newton K, Caceres JF, Dubus P, Malumbres M, Barbacid M. Cdk1 is sufficient to drive the mammalian cell cycle Nature 2007. 448 811– 815 - PubMed
-
- Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, Barbero JL, Malumbres M, Barbacid M. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice Nat Genet 2003. 35 25– 31 - PubMed
-
- Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. Cdk2 knockout mice are viable Curr Biol 2003. 13 1775– 1785 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

