Bisphenol A and Related Alkylphenols Exert Nongenomic Estrogenic Actions Through a G Protein-Coupled Estrogen Receptor 1 (Gper)/Epidermal Growth Factor Receptor (Egfr) Pathway to Inhibit Meiotic Maturation of Zebrafish Oocytes
- PMID: 26490843
- PMCID: PMC4712694
- DOI: 10.1095/biolreprod.115.132316
Bisphenol A and Related Alkylphenols Exert Nongenomic Estrogenic Actions Through a G Protein-Coupled Estrogen Receptor 1 (Gper)/Epidermal Growth Factor Receptor (Egfr) Pathway to Inhibit Meiotic Maturation of Zebrafish Oocytes
Abstract
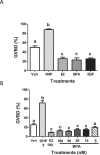
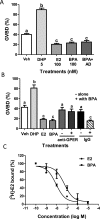
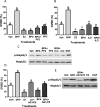
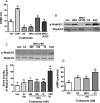
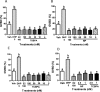
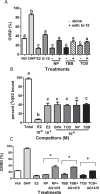
Xenobiotic estrogens, such as bisphenol A (BPA), disrupt a wide variety of genomic estrogen actions, but their nongenomic estrogen actions remain poorly understood. We investigated nongenomic estrogenic effects of low concentrations of BPA and three related alkylphenols on the inhibition of zebrafish oocye maturation (OM) mediated through a G protein-coupled estrogen receptor 1 (Gper)-dependent epidermal growth factor receptor (Egfr) pathway. BPA (10-100 nM) treatment for 3 h mimicked the effects of estradiol-17beta (E2) and EGF, decreasing spontaneous maturation of defolliculated zebrafish oocytes, an effect not blocked by coincubation with actinomycin D, but blocked by coincubation with a Gper antibody. BPA displayed relatively high binding affinity (15.8% that of E2) for recombinant zebrafish Gper. The inhibitory effects of BPA were attenuated by inhibition of upstream regulators of Egfr, intracellular tyrosine kinase (Src) with PP2, and matrix metalloproteinase with ilomastat. Treatment with an inhibitor of Egfr transactivation, AG1478, and an inhibitor of the mitogen-activated protein kinase (MAPK) 3/1 pathway, U0126, increased spontaneous OM and blocked the inhibitory effects of BPA, E2, and the selective GPER agonist, G-1. Western blot analysis showed that BPA (10-200 nM) mimicked the stimulatory effects of E2 and EGF on Mapk3/1 phosphorylation. Tetrabromobisphenol A, 4-nonylphenol, and tetrachlorobisphenol A (5-100 nM) also inhibited OM, an effect blocked by cotreatment with AG1478, as well as with the GPER antagonist, G-15, and displayed similar binding affinities as BPA to zebrafish Gper. The results suggest that BPA and related alkylphenols disrupt zebrafish OM by a novel nongenomic estrogenic mechanism involving activation of the Gper/Egfr/Mapk3/1 pathway.
Keywords: EGFR; GPER; GPR30; MAPkinase; bisphenol A; endocrine disruptors; meiotic arrest; nonylphenol; oocyte maturation; tetrabromobisphenol A; tetrachorobisphenol A; zebrafish.
© 2015 by the Society for the Study of Reproduction, Inc.
Figures
Similar articles
-
Involvement of epidermal growth factor receptor signaling in estrogen inhibition of oocyte maturation mediated through the G protein-coupled estrogen receptor (Gper) in zebrafish (Danio rerio).Biol Reprod. 2011 Jul;85(1):42-50. doi: 10.1095/biolreprod.110.088765. Epub 2011 Feb 23. Biol Reprod. 2011. PMID: 21349822 Free PMC article.
-
Role of G-protein-coupled estrogen receptor (GPER/GPR30) in maintenance of meiotic arrest in fish oocytes.J Steroid Biochem Mol Biol. 2017 Mar;167:153-161. doi: 10.1016/j.jsbmb.2016.12.005. Epub 2016 Dec 19. J Steroid Biochem Mol Biol. 2017. PMID: 28007532 Review.
-
The catecholestrogen, 2-hydroxyestradiol-17beta, acts as a G protein-coupled estrogen receptor 1 (GPER/GPR30) antagonist to promote the resumption of meiosis in zebrafish oocytes.Biol Reprod. 2015 Mar;92(3):69. doi: 10.1095/biolreprod.114.125674. Epub 2015 Jan 21. Biol Reprod. 2015. PMID: 25609836
-
Reprint of "Role of G protein-coupled estrogen receptor (GPER/GPR30) in maintenance of meiotic arrest in fish oocytes".J Steroid Biochem Mol Biol. 2018 Feb;176:23-30. doi: 10.1016/j.jsbmb.2017.10.023. Epub 2017 Nov 2. J Steroid Biochem Mol Biol. 2018. PMID: 29102625 Review.
-
Role of natriuretic peptide receptor 2-mediated signaling in meiotic arrest of zebrafish oocytes and its estrogen regulation through G protein-coupled estrogen receptor (Gper).Gen Comp Endocrinol. 2018 Sep 1;265:180-187. doi: 10.1016/j.ygcen.2018.03.024. Epub 2018 Mar 22. Gen Comp Endocrinol. 2018. PMID: 29574150
Cited by
-
Editor's Highlight: Transcriptome Profiling Reveals Bisphenol A Alternatives Activate Estrogen Receptor Alpha in Human Breast Cancer Cells.Toxicol Sci. 2017 Aug 1;158(2):431-443. doi: 10.1093/toxsci/kfx101. Toxicol Sci. 2017. PMID: 28591870 Free PMC article.
-
Identification of Novel MicroRNAs and Characterization of MicroRNA Expression Profiles in Zebrafish Ovarian Follicular Cells.Front Endocrinol (Lausanne). 2019 Jul 31;10:518. doi: 10.3389/fendo.2019.00518. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31417497 Free PMC article.
-
Overview of the Mechanisms of Action of Selected Bisphenols and Perfluoroalkyl Chemicals on the Male Reproductive Axes.Front Genet. 2021 Sep 27;12:692897. doi: 10.3389/fgene.2021.692897. eCollection 2021. Front Genet. 2021. PMID: 34646297 Free PMC article. Review.
-
Associations Between Behavioral Effects of Bisphenol A and DNA Methylation in Zebrafish Embryos.Front Genet. 2019 Mar 8;10:184. doi: 10.3389/fgene.2019.00184. eCollection 2019. Front Genet. 2019. PMID: 30906313 Free PMC article.
-
Endocrine-disrupting chemicals in aquatic environment: what are the risks for fish gametes?Fish Physiol Biochem. 2018 Dec;44(6):1561-1576. doi: 10.1007/s10695-018-0507-z. Epub 2018 Jun 11. Fish Physiol Biochem. 2018. PMID: 29948447 Review.
References
-
- Loomis AK, Thomas P. Binding characteristics of estrogen receptor (ER) in Atlantic croaker (Micropogonias undulatus) testis: different affinity for estrogens and xenobiotics from that of hepatic ER Biol Reprod 1999. 61 51– 60 - PubMed
-
- Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, Tong W, Shi L, Perkins R, Sheehan DM. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands Toxicol Sci 2000. 54 1: 138– 153 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

