Hippocampal Volume Reduction in Humans Predicts Impaired Allocentric Spatial Memory in Virtual-Reality Navigation
- PMID: 26490854
- PMCID: PMC4683681
- DOI: 10.1523/JNEUROSCI.0801-15.2015
Hippocampal Volume Reduction in Humans Predicts Impaired Allocentric Spatial Memory in Virtual-Reality Navigation
Abstract
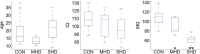
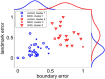
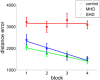
The extent to which navigational spatial memory depends on hippocampal integrity in humans is not well documented. We investigated allocentric spatial recall using a virtual environment in a group of patients with severe hippocampal damage (SHD), a group of patients with "moderate" hippocampal damage (MHD), and a normal control group. Through four learning blocks with feedback, participants learned the target locations of four different objects in a circular arena. Distal cues were present throughout the experiment to provide orientation. A circular boundary as well as an intra-arena landmark provided spatial reference frames. During a subsequent test phase, recall of all four objects was tested with only the boundary or the landmark being present. Patients with SHD were impaired in both phases of this task. Across groups, performance on both types of spatial recall was highly correlated with memory quotient (MQ), but not with intelligence quotient (IQ), age, or sex. However, both measures of spatial recall separated experimental groups beyond what would be expected based on MQ, a widely used measure of general memory function. Boundary-based and landmark-based spatial recall were both strongly related to bilateral hippocampal volumes, but not to volumes of the thalamus, putamen, pallidum, nucleus accumbens, or caudate nucleus. The results show that boundary-based and landmark-based allocentric spatial recall are similarly impaired in patients with SHD, that both types of recall are impaired beyond that predicted by MQ, and that recall deficits are best explained by a reduction in bilateral hippocampal volumes.
Significance statement: In humans, bilateral hippocampal atrophy can lead to profound impairments in episodic memory. Across species, perhaps the most well-established contribution of the hippocampus to memory is not to episodic memory generally but to allocentric spatial memory. However, the extent to which navigational spatial memory depends on hippocampal integrity in humans is not well documented. We investigated spatial recall using a virtual environment in two groups of patients with hippocampal damage (moderate/severe) and a normal control group. The results showed that patients with severe hippocampal damage are impaired in learning and recalling allocentric spatial information. Furthermore, hippocampal volume reduction impaired allocentric navigation beyond what can be predicted by memory quotient as a widely used measure of general memory function.
Keywords: amnesia; human; memory; navigation; spatial; virtual reality.
Copyright © 2015 Guderian et al.
Figures


Comment in
-
The Hippocampus Contributes to Allocentric Spatial Memory through Coherent Scene Representations.J Neurosci. 2016 Mar 2;36(9):2555-7. doi: 10.1523/JNEUROSCI.4548-15.2016. J Neurosci. 2016. PMID: 26936996 Free PMC article. No abstract available.
Similar articles
-
The Hippocampus Contributes to Allocentric Spatial Memory through Coherent Scene Representations.J Neurosci. 2016 Mar 2;36(9):2555-7. doi: 10.1523/JNEUROSCI.4548-15.2016. J Neurosci. 2016. PMID: 26936996 Free PMC article. No abstract available.
-
Egocentric memory impaired and allocentric memory intact as assessed by virtual reality in subjects with unilateral parietal cortex lesions.Neuropsychologia. 2009 Jan;47(1):59-69. doi: 10.1016/j.neuropsychologia.2008.08.018. Epub 2008 Aug 22. Neuropsychologia. 2009. PMID: 18789955
-
Close but no cigar: Spatial precision deficits following medial temporal lobe lesions provide novel insight into theoretical models of navigation and memory.Hippocampus. 2018 Jan;28(1):31-41. doi: 10.1002/hipo.22801. Epub 2017 Sep 26. Hippocampus. 2018. PMID: 28888032 Free PMC article.
-
Spatial representations in the primate hippocampus, and their functions in memory and navigation.Prog Neurobiol. 2018 Dec;171:90-113. doi: 10.1016/j.pneurobio.2018.09.004. Epub 2018 Sep 13. Prog Neurobiol. 2018. PMID: 30219248 Review.
-
Egocentric and Allocentric Spatial Memory in Mild Cognitive Impairment with Real-World and Virtual Navigation Tasks: A Systematic Review.J Alzheimers Dis. 2021;79(1):95-116. doi: 10.3233/JAD-201017. J Alzheimers Dis. 2021. PMID: 33216034 Free PMC article.
Cited by
-
Altered structure and functional connectivity of the hippocampus are associated with social and mathematical difficulties in nonverbal learning disability.Hippocampus. 2021 Jan;31(1):79-88. doi: 10.1002/hipo.23264. Epub 2020 Sep 19. Hippocampus. 2021. PMID: 32949475 Free PMC article.
-
Prenatal Exposure to Air Pollution and Early-Life Stress Effects on Hippocampal Subregional Volumes and Associations With Visuospatial Reasoning.Biol Psychiatry Glob Open Sci. 2022 Jul;2(3):292-300. doi: 10.1016/j.bpsgos.2022.05.003. Epub 2022 May 30. Biol Psychiatry Glob Open Sci. 2022. PMID: 35978944 Free PMC article.
-
Spatial Navigation and Visuospatial Strategies in Typical and Atypical Aging.Brain Sci. 2021 Oct 27;11(11):1421. doi: 10.3390/brainsci11111421. Brain Sci. 2021. PMID: 34827423 Free PMC article.
-
A bedside application-based assessment of spatial orientation and memory: approaches and lessons learned.J Neurol. 2019 Sep;266(Suppl 1):126-138. doi: 10.1007/s00415-019-09409-7. Epub 2019 Jun 25. J Neurol. 2019. PMID: 31240446 Free PMC article.
-
The Role of Virtual Reality in Screening, Diagnosing, and Rehabilitating Spatial Memory Deficits.Front Hum Neurosci. 2021 Feb 5;15:628818. doi: 10.3389/fnhum.2021.628818. eCollection 2021. Front Hum Neurosci. 2021. PMID: 33613216 Free PMC article.
References
-
- Caliński T, Harabasz J. A dendrite method for cluster analysis. Commun Stat. 1974;3:1–27. doi: 10.1080/03610917408548446. - DOI
-
- Cohen M. Children's Memory Scale. San Antonio, TX: Harcourt Brace and Co; 1997.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
