The Periaqueductal Gray Orchestrates Sensory and Motor Circuits at Multiple Levels of the Neuraxis
- PMID: 26490855
- PMCID: PMC4683682
- DOI: 10.1523/JNEUROSCI.0261-15.2015
The Periaqueductal Gray Orchestrates Sensory and Motor Circuits at Multiple Levels of the Neuraxis
Abstract
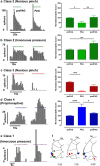
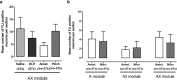
The periaqueductal gray (PAG) coordinates behaviors essential to survival, including striking changes in movement and posture (e.g., escape behaviors in response to noxious stimuli vs freezing in response to fear-evoking stimuli). However, the neural circuits underlying the expression of these behaviors remain poorly understood. We demonstrate in vivo in rats that activation of the ventrolateral PAG (vlPAG) affects motor systems at multiple levels of the neuraxis through the following: (1) differential control of spinal neurons that forward sensory information to the cerebellum via spino-olivo-cerebellar pathways (nociceptive signals are reduced while proprioceptive signals are enhanced); (2) alterations in cerebellar nuclear output as revealed by changes in expression of Fos-like immunoreactivity; and (3) regulation of spinal reflex circuits, as shown by an increase in α-motoneuron excitability. The capacity to coordinate sensory and motor functions is demonstrated in awake, behaving rats, in which natural activation of the vlPAG in fear-conditioned animals reduced transmission in spino-olivo-cerebellar pathways during periods of freezing that were associated with increased muscle tone and thus motor outflow. The increase in spinal motor reflex excitability and reduction in transmission of ascending sensory signals via spino-olivo-cerebellar pathways occurred simultaneously. We suggest that the interactions revealed in the present study between the vlPAG and sensorimotor circuits could form the neural substrate for survival behaviors associated with vlPAG activation.
Significance statement: Neural circuits that coordinate survival behaviors remain poorly understood. We demonstrate in rats that the periaqueductal gray (PAG) affects motor systems at the following multiple levels of the neuraxis: (1) through altering transmission in spino-olivary pathways that forward sensory signals to the cerebellum, reducing and enhancing transmission of nociceptive and proprioceptive information, respectively; (2) by alterations in cerebellar output; and (3) through enhancement of spinal motor reflex pathways. The sensory and motor effects occurred at the same time and were present in both anesthetized animals and behavioral experiments in which fear conditioning naturally activated the PAG. The results provide insights into the neural circuits that enable an animal to be ready and able to react to danger, thus assisting in survival.
Keywords: cerebellum; fear; nociception; periaqueductal grey; proprioception; spinal cord.
Copyright © 2015 Koutsikou, Watson et al.
Figures





Similar articles
-
Neural substrates underlying fear-evoked freezing: the periaqueductal grey-cerebellar link.J Physiol. 2014 May 15;592(10):2197-213. doi: 10.1113/jphysiol.2013.268714. Epub 2014 Mar 17. J Physiol. 2014. PMID: 24639484 Free PMC article.
-
Fos-like immunoreactive neurons following electrical stimulation of the dorsal periaqueductal gray at freezing and escape thresholds.Brain Res Bull. 2003 Dec 30;62(3):179-89. doi: 10.1016/j.brainresbull.2003.09.010. Brain Res Bull. 2003. PMID: 14698351
-
Cerebellar modulation of memory encoding in the periaqueductal grey and fear behaviour.Elife. 2022 Mar 15;11:e76278. doi: 10.7554/eLife.76278. Elife. 2022. PMID: 35287795 Free PMC article.
-
Top down control of spinal sensorimotor circuits essential for survival.J Physiol. 2017 Jul 1;595(13):4151-4158. doi: 10.1113/JP273360. Epub 2017 Apr 24. J Physiol. 2017. PMID: 28294351 Free PMC article. Review.
-
The olivo-cerebellar system and its relationship to survival circuits.Front Neural Circuits. 2013 Apr 23;7:72. doi: 10.3389/fncir.2013.00072. eCollection 2013. Front Neural Circuits. 2013. PMID: 23630468 Free PMC article. Review.
Cited by
-
Circuits for State-Dependent Modulation of Locomotion.Front Hum Neurosci. 2021 Nov 10;15:745689. doi: 10.3389/fnhum.2021.745689. eCollection 2021. Front Hum Neurosci. 2021. PMID: 34858153 Free PMC article. Review.
-
Imbalance of flight-freeze responses and their cellular correlates in the Nlgn3-/y rat model of autism.Mol Autism. 2022 Jul 18;13(1):34. doi: 10.1186/s13229-022-00511-8. Mol Autism. 2022. PMID: 35850732 Free PMC article.
-
Fastigial nuclei surgical damage and focal midbrain disruption implicate PAG survival circuits in cerebellar mutism syndrome.Neuro Oncol. 2023 Feb 14;25(2):375-385. doi: 10.1093/neuonc/noac168. Neuro Oncol. 2023. PMID: 35789275 Free PMC article.
-
Central Sensitization-Based Classification for Temporomandibular Disorders: A Pathogenetic Hypothesis.Pain Res Manag. 2017;2017:5957076. doi: 10.1155/2017/5957076. Epub 2017 Aug 28. Pain Res Manag. 2017. PMID: 28932132 Free PMC article. Review.
-
Baclofen pump with pre-brainstem catheter tip placement: technical note and case series.Childs Nerv Syst. 2021 Jan;37(1):203-210. doi: 10.1007/s00381-020-04679-3. Epub 2020 Jun 6. Childs Nerv Syst. 2021. PMID: 32504173
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
