A Post-Docking Role of Synaptotagmin 1-C2B Domain Bottom Residues R398/399 in Mouse Chromaffin Cells
- PMID: 26490858
- PMCID: PMC6605428
- DOI: 10.1523/JNEUROSCI.1911-15.2015
A Post-Docking Role of Synaptotagmin 1-C2B Domain Bottom Residues R398/399 in Mouse Chromaffin Cells
Abstract
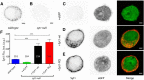
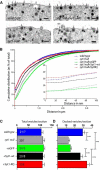
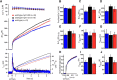
Synaptotagmin-1 (Syt1) is the principal Ca(2+) sensor for vesicle fusion and is also essential for vesicle docking in chromaffin cells. Docking depends on interactions of the Syt1-C2B domain with the t-SNARE SNAP25/Syntaxin1 complex and/or plasma membrane phospholipids. Here, we investigated the role of the positively charged "bottom" region of the C2B domain, proposed to help crosslink membranes, in vesicle docking and secretion in mouse chromaffin cells and in cell-free assays. We expressed a double mutation shown previously to interfere with lipid mixing between proteoliposomes and with synaptic transmission, Syt1-R398/399Q (RQ), in syt1 null mutant cells. Ultrastructural morphometry revealed that Syt1-RQ fully restored the docking defect observed previously in syt1 null mutant cells, similar to wild type Syt1 (Syt1-wt). Small unilamellar lipid vesicles (SUVs) that contained the v-SNARE Synaptobrevin2 and Syt1-R398/399Q also docked to t-SNARE-containing giant vesicles (GUVs), similar to Syt1-wt. However, unlike Syt1-wt, Syt1-RQ-induced docking was strictly PI(4,5)P2-dependent. Unlike docking, neither synchronized secretion in chromaffin cells nor Ca(2+)-triggered SUV-GUV fusion was restored by the Syt1 mutants. Finally, overexpressing the RQ-mutant in wild type cells produced no effect on either docking or secretion. We conclude that the positively charged bottom region in the C2B domain--and, by inference, Syt1-mediated membrane crosslinking--is required for triggering fusion, but not for docking. Secretory vesicles dock by multiple, PI(4,5)P2-dependent and PI(4,5)P2-independent mechanisms. The R398/399 mutations selectively disrupt the latter and hereby help to discriminate protein regions involved in different aspects of Syt1 function in docking and fusion.
Significance statement: This study provides new insights in how the two opposite sides of the C2B domain of Synaptotagmin-1 participate in secretory vesicle fusion, and in more upstream steps, especially vesicle docking. We show that the "bottom" surface of the C2B domain is required for triggering fusion, but not for docking. Synaptotagmin-1 promotes docking by multiple, PI(4,5)P2-dependent and PI(4,5)P2-independent mechanisms. Mutations in the C2B bottom surface (R398/399) selectively disrupt the latter. These mutations help to discriminate protein regions involved in different aspects of Synaptotagmin-1 function in docking and fusion.
Keywords: mouse chromaffin cells; patch-clamp technique; synaptotagmin-1; ultrastructural analysis.
Copyright © 2015 the authors 0270-6474/15/3514173-11$15.00/0.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Genetic analysis of synaptotagmin C2 domain specificity in regulating spontaneous and evoked neurotransmitter release.J Neurosci. 2013 Jan 2;33(1):187-200. doi: 10.1523/JNEUROSCI.3214-12.2013. J Neurosci. 2013. PMID: 23283333 Free PMC article.
-
Synaptotagmin-1 drives synchronous Ca2+-triggered fusion by C2B-domain-mediated synaptic-vesicle-membrane attachment.Nat Neurosci. 2018 Jan;21(1):33-40. doi: 10.1038/s41593-017-0037-5. Epub 2017 Dec 11. Nat Neurosci. 2018. PMID: 29230057 Free PMC article.
-
Synaptotagmin-1 C2B domain interacts simultaneously with SNAREs and membranes to promote membrane fusion.Elife. 2016 Apr 15;5:e14211. doi: 10.7554/eLife.14211. Elife. 2016. PMID: 27083046 Free PMC article.
-
Models of synaptotagmin-1 to trigger Ca2+ -dependent vesicle fusion.FEBS Lett. 2018 Nov;592(21):3480-3492. doi: 10.1002/1873-3468.13193. Epub 2018 Jul 30. FEBS Lett. 2018. PMID: 30004579 Review.
-
Synaptotagmin-1: A Multi-Functional Protein that Mediates Vesicle Docking, Priming, and Fusion.Curr Protein Pept Sci. 2021;22(6):470-478. doi: 10.2174/1389203722666210325110231. Curr Protein Pept Sci. 2021. PMID: 33823763 Review.
Cited by
-
Mutations of Single Residues in the Complexin N-terminus Exhibit Distinct Phenotypes in Synaptic Vesicle Fusion.J Neurosci. 2024 Jul 31;44(31):e0076242024. doi: 10.1523/JNEUROSCI.0076-24.2024. J Neurosci. 2024. PMID: 38951039 Free PMC article.
-
Synaptotagmin-1 enables frequency coding by suppressing asynchronous release in a temperature dependent manner.Sci Rep. 2019 Aug 5;9(1):11341. doi: 10.1038/s41598-019-47487-9. Sci Rep. 2019. PMID: 31383906 Free PMC article.
-
SNAP25 disease mutations change the energy landscape for synaptic exocytosis due to aberrant SNARE interactions.Elife. 2024 Feb 27;12:RP88619. doi: 10.7554/eLife.88619. Elife. 2024. PMID: 38411501 Free PMC article.
-
Mechanism of neurotransmitter release coming into focus.Protein Sci. 2018 Aug;27(8):1364-1391. doi: 10.1002/pro.3445. Epub 2018 Jul 10. Protein Sci. 2018. PMID: 29893445 Free PMC article. Review.
-
Pull-down combined with proteomic strategy reveals functional diversity of synaptotagmin I.PeerJ. 2017 Feb 8;5:e2973. doi: 10.7717/peerj.2973. eCollection 2017. PeerJ. 2017. PMID: 28194317 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
