Internalized Tau Oligomers Cause Neurodegeneration by Inducing Accumulation of Pathogenic Tau in Human Neurons Derived from Induced Pluripotent Stem Cells
- PMID: 26490863
- PMCID: PMC6605424
- DOI: 10.1523/JNEUROSCI.1523-15.2015
Internalized Tau Oligomers Cause Neurodegeneration by Inducing Accumulation of Pathogenic Tau in Human Neurons Derived from Induced Pluripotent Stem Cells
Abstract
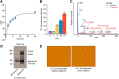
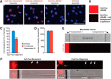
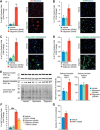
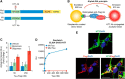
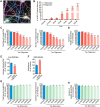
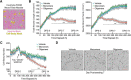
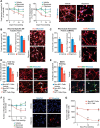
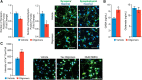
Neuronal inclusions of hyperphosphorylated and aggregated tau protein are a pathological hallmark of several neurodegenerative tauopathies, including Alzheimer's disease (AD). The hypothesis of tau transmission in AD has emerged from histopathological studies of the spatial and temporal progression of tau pathology in postmortem patient brains. Increasing evidence in cellular and animal models supports the phenomenon of intercellular spreading of tau. However, the molecular and cellular mechanisms of pathogenic tau transmission remain unknown. The studies described herein investigate tau pathology propagation using human neurons derived from induced pluripotent stem cells. Neurons were seeded with full-length human tau monomers and oligomers and chronic effects on neuronal viability and function were examined over time. Tau oligomer-treated neurons exhibited an increase in aggregated and phosphorylated pathological tau. These effects were associated with neurite retraction, loss of synapses, aberrant calcium homeostasis, and imbalanced neurotransmitter release. In contrast, tau monomer treatment did not produce any measureable changes. This work supports the hypothesis that tau oligomers are toxic species that can drive the spread of tau pathology and neurodegeneration.
Significance statement: Several independent studies have implicated tau protein as central to Alzheimer's disease progression and cell-to-cell pathology propagation. In this study, we investigated the ability of different tau species to propagate pathology in human neurons derived from induced pluripotent stem cells, which to date has not been shown. We demonstrated that tau oligomers, but not monomers, induce accumulation of pathological, hyperphosphorylated tau. This effect was accompanied with neurite degeneration, loss of synapses, aberrant calcium homeostasis, imbalanced neurotransmitter release, and ultimately with neuronal death. This study bridges various tau pathological phenotypes into a single and relevant induced pluripotent stem cell neuronal model of human disease that can be applied to the discovery of the mechanisms of tau-induced neurodegeneration.
Keywords: Alzheimer's disease; hiPSC neurons; neurodegeneration; pathology propagation; tau oligomer seeds.
Copyright © 2015 the authors 0270-6474/15/3514234-17$15.00/0.
Figures
Similar articles
-
Continuous Monitoring of Tau-Induced Neurotoxicity in Patient-Derived iPSC-Neurons.J Neurosci. 2021 May 12;41(19):4335-4348. doi: 10.1523/JNEUROSCI.2590-20.2021. Epub 2021 Apr 23. J Neurosci. 2021. PMID: 33893219 Free PMC article.
-
Generation of a human induced pluripotent stem cell-based model for tauopathies combining three microtubule-associated protein TAU mutations which displays several phenotypes linked to neurodegeneration.Alzheimers Dement. 2018 Oct;14(10):1261-1280. doi: 10.1016/j.jalz.2018.05.007. Epub 2018 Jul 20. Alzheimers Dement. 2018. PMID: 30036493
-
GFP-Mutant Human Tau Transgenic Mice Develop Tauopathy Following CNS Injections of Alzheimer's Brain-Derived Pathological Tau or Synthetic Mutant Human Tau Fibrils.J Neurosci. 2017 Nov 22;37(47):11485-11494. doi: 10.1523/JNEUROSCI.2393-17.2017. Epub 2017 Oct 6. J Neurosci. 2017. PMID: 28986461 Free PMC article.
-
[Significance of tau in the development of Alzheimer's disease].Brain Nerve. 2010 Jul;62(7):701-8. Brain Nerve. 2010. PMID: 20675874 Review. Japanese.
-
Nature of Tau-Associated Neurodegeneration and the Molecular Mechanisms.J Alzheimers Dis. 2018;62(3):1305-1317. doi: 10.3233/JAD-170788. J Alzheimers Dis. 2018. PMID: 29562535 Free PMC article. Review.
Cited by
-
Editorial: Protein misfolding, altered mechanisms and neurodegeneration.Front Mol Neurosci. 2023 Feb 1;16:1134855. doi: 10.3389/fnmol.2023.1134855. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36818654 Free PMC article. No abstract available.
-
Binding and neurotoxicity mitigation of toxic tau oligomers by synthetic heparin like oligosaccharides.Chem Commun (Camb). 2018 Sep 6;54(72):10120-10123. doi: 10.1039/c8cc05072d. Chem Commun (Camb). 2018. PMID: 30128457 Free PMC article.
-
Induced pluripotent stem cell-based organ-on-a-chip as personalized drug screening tools: A focus on neurodegenerative disorders.J Tissue Eng. 2022 May 9;13:20417314221095339. doi: 10.1177/20417314221095339. eCollection 2022 Jan-Dec. J Tissue Eng. 2022. PMID: 35570845 Free PMC article. Review.
-
On the role of the cellular prion protein in the uptake and signaling of pathological aggregates in neurodegenerative diseases.Prion. 2020 Dec;14(1):257-270. doi: 10.1080/19336896.2020.1854034. Prion. 2020. PMID: 33345731 Free PMC article. Review.
-
Distinct Conformations, Aggregation and Cellular Internalization of Different Tau Strains.Front Cell Neurosci. 2019 Jul 9;13:296. doi: 10.3389/fncel.2019.00296. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31338022 Free PMC article.
References
-
- Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, Schmitt I, Wullner U, Evert BO, O'Kane CJ, Rubinsztein DC. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15:433–442. - PubMed
-
- Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, Yue M, Wszolek Z, Ashe K, Knight J, Dickson D, Andorfer C, Rosenberry TL, Lewis J, Hutton M, Janus C. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci. 2007;27:3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
