Metabolic Connection of Inflammatory Pain: Pivotal Role of a Pyruvate Dehydrogenase Kinase-Pyruvate Dehydrogenase-Lactic Acid Axis
- PMID: 26490872
- PMCID: PMC6605420
- DOI: 10.1523/JNEUROSCI.1910-15.2015
Metabolic Connection of Inflammatory Pain: Pivotal Role of a Pyruvate Dehydrogenase Kinase-Pyruvate Dehydrogenase-Lactic Acid Axis
Abstract
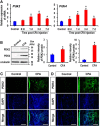
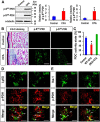
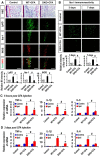
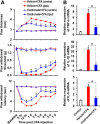
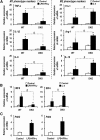
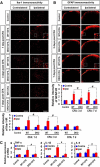
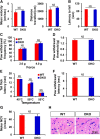
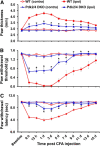
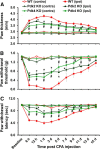
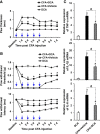
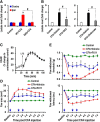
Pyruvate dehydrogenase kinases (PDK1-4) are mitochondrial metabolic regulators that serve as decision makers via modulation of pyruvate dehydrogenase (PDH) activity to convert pyruvate either aerobically to acetyl-CoA or anaerobically to lactate. Metabolic dysregulation and inflammatory processes are two sides of the same coin in several pathophysiological conditions. The lactic acid surge associated with the metabolic shift has been implicated in diverse painful states. In this study, we investigated the role of PDK-PDH-lactic acid axis in the pathogenesis of chronic inflammatory pain. Deficiency of Pdk2 and/or Pdk4 in mice attenuated complete Freund's adjuvant (CFA)-induced pain hypersensitivities. Likewise, Pdk2/4 deficiency attenuated the localized lactic acid surge along with hallmarks of peripheral and central inflammation following intraplantar administration of CFA. In vitro studies supported the role of PDK2/4 as promoters of classical proinflammatory activation of macrophages. Moreover, the pharmacological inhibition of PDKs or lactic acid production diminished CFA-induced inflammation and pain hypersensitivities. Thus, a PDK-PDH-lactic acid axis seems to mediate inflammation-driven chronic pain, establishing a connection between metabolism and inflammatory pain.
Significance statement: The mitochondrial pyruvate dehydrogenase (PDH) kinases (PDKs) and their substrate PDH orchestrate the conversion of pyruvate either aerobically to acetyl-CoA or anaerobically to lactate. Lactate, the predominant end product of glycolysis, has recently been identified as a signaling molecule for neuron-glia interactions and neuronal plasticity. Pathological metabolic shift and subsequent lactic acid production are thought to play an important role in diverse painful states; however, their contribution to inflammation-driven pain is still to be comprehended. Here, we report that the PDK-PDH-lactic acid axis constitutes a key component of inflammatory pain pathogenesis. Our findings establish an unanticipated link between metabolism and inflammatory pain. This study unlocks a previously ill-explored research avenue for the metabolic control of inflammatory pain pathogenesis.
Keywords: PDK-PDH-lactic acid axis; chronic inflammatory pain; inflammation; macrophages; metabolism; pain.
Copyright © 2015 the authors 0270-6474/15/3514354-17$15.00/0.
Figures

Similar articles
-
Pyruvate Dehydrogenase Kinase-mediated Glycolytic Metabolic Shift in the Dorsal Root Ganglion Drives Painful Diabetic Neuropathy.J Biol Chem. 2016 Mar 11;291(11):6011-6025. doi: 10.1074/jbc.M115.699215. Epub 2016 Jan 14. J Biol Chem. 2016. PMID: 26769971 Free PMC article.
-
Metabolic reprogramming by the pyruvate dehydrogenase kinase-lactic acid axis: Linking metabolism and diverse neuropathophysiologies.Neurosci Biobehav Rev. 2016 Sep;68:1-19. doi: 10.1016/j.neubiorev.2016.05.006. Epub 2016 May 11. Neurosci Biobehav Rev. 2016. PMID: 27179453 Review.
-
Pyruvate dehydrogenase kinase 2 and 4 gene deficiency attenuates nociceptive behaviors in a mouse model of acute inflammatory pain.J Neurosci Res. 2016 Sep;94(9):837-49. doi: 10.1002/jnr.23727. Epub 2016 Mar 1. J Neurosci Res. 2016. PMID: 26931482
-
Pro-inflammatory Macrophages Sustain Pyruvate Oxidation through Pyruvate Dehydrogenase for the Synthesis of Itaconate and to Enable Cytokine Expression.J Biol Chem. 2016 Feb 19;291(8):3932-46. doi: 10.1074/jbc.M115.676817. Epub 2015 Dec 17. J Biol Chem. 2016. PMID: 26679997 Free PMC article.
-
Therapeutic potential of the mammalian pyruvate dehydrogenase kinases in the prevention of hyperglycaemia.Curr Drug Targets Immune Endocr Metabol Disord. 2002 Jul;2(2):151-65. Curr Drug Targets Immune Endocr Metabol Disord. 2002. PMID: 12476789 Review.
Cited by
-
Pyruvate dehydrogenase kinase regulates macrophage polarization in metabolic and inflammatory diseases.Front Immunol. 2023 Dec 18;14:1296687. doi: 10.3389/fimmu.2023.1296687. eCollection 2023. Front Immunol. 2023. PMID: 38193078 Free PMC article. Review.
-
Amyotrophic Lateral Sclerosis Pathoetiology and Pathophysiology: Roles of Astrocytes, Gut Microbiome, and Muscle Interactions via the Mitochondrial Melatonergic Pathway, with Disruption by Glyphosate-Based Herbicides.Int J Mol Sci. 2022 Dec 29;24(1):587. doi: 10.3390/ijms24010587. Int J Mol Sci. 2022. PMID: 36614029 Free PMC article. Review.
-
Astrocytes in the Ventrolateral Preoptic Area Promote Sleep.J Neurosci. 2020 Nov 18;40(47):8994-9011. doi: 10.1523/JNEUROSCI.1486-20.2020. Epub 2020 Oct 16. J Neurosci. 2020. PMID: 33067363 Free PMC article.
-
PANoptosis-based molecular subtyping and HPAN-index predicts therapeutic response and survival in hepatocellular carcinoma.Front Immunol. 2023 Jun 15;14:1197152. doi: 10.3389/fimmu.2023.1197152. eCollection 2023. Front Immunol. 2023. PMID: 37398672 Free PMC article.
-
Hyperpolarized 13C MR metabolic imaging can detect neuroinflammation in vivo in a multiple sclerosis murine model.Proc Natl Acad Sci U S A. 2017 Aug 15;114(33):E6982-E6991. doi: 10.1073/pnas.1613345114. Epub 2017 Jul 31. Proc Natl Acad Sci U S A. 2017. PMID: 28760957 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
