Nurr1 and Retinoid X Receptor Ligands Stimulate Ret Signaling in Dopamine Neurons and Can Alleviate α-Synuclein Disrupted Gene Expression
- PMID: 26490873
- PMCID: PMC6605427
- DOI: 10.1523/JNEUROSCI.1155-15.2015
Nurr1 and Retinoid X Receptor Ligands Stimulate Ret Signaling in Dopamine Neurons and Can Alleviate α-Synuclein Disrupted Gene Expression
Abstract
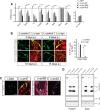
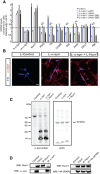
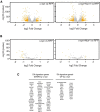
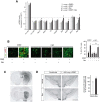
α-synuclein, a protein enriched in Lewy bodies and highly implicated in neurotoxicity in Parkinson's disease, is distributed both at nerve terminals and in the cell nucleus. Here we show that a nuclear derivative of α-synuclein induces more pronounced changes at the gene expression level in mouse primary dopamine (DA) neurons compared to a derivative that is excluded from the nucleus. Moreover, by RNA sequencing we analyzed the extent of genome-wide effects on gene expression resulting from expression of human α-synuclein in primary mouse DA neurons. The results implicated the transcription factor Nurr1 as a key dysregulated target of α-synuclein toxicity. Forced Nurr1 expression restored the expression of hundreds of dysregulated genes in primary DA neurons expressing α-synuclein, and therefore prompted us to test the possibility that Nurr1 can be pharmacologically targeted by bexarotene, a ligand for the retinoid X receptor that forms heterodimers with Nurr1. Although our data demonstrated that bexarotene was ineffective in neuroprotection in rats in vivo, the results revealed that bexarotene has the capacity to coregulate subsets of Nurr1 target genes including the receptor tyrosine kinase subunit Ret. Moreover, bexarotene was able to restore dysfunctional Ret-dependent neurotrophic signaling in α-synuclein-overexpressing mouse DA neurons. These data highlight the role of the Nurr1-Ret signaling pathway as a target of α-synuclein toxicity and suggest that retinoid X receptor ligands with appropriate pharmacological properties could have therapeutic potential in Parkinson's disease.
Significance statement: How α-synuclein, a protein enriched in Lewy bodies in Parkinson's disease, is causing neuropathology in dopamine neurons remains unclear. This study elucidated how α-synuclein is influencing gene expression and how Nurr1, a transcription factor known to protect dopamine neurons against α-synuclein toxicity, can counteract these effects. Moreover, given the protective role of Nurr1, this study also investigated how Nurr1 could be pharmacologically targeted via bexarotene, a ligand of Nurr1's heterodimerization partner retinoid X receptor (RXR). The results showed that RXR ligands could increase neurotrophic signaling, but provided a mixed picture of its potential in a Parkinson's disease rat model in vivo. However, this study clearly emphasized Nurr1's neuroprotective role and indicated that other RXR ligands could have therapeutic potential in Parkinson's disease.
Copyright © 2015 the authors 0270-6474/15/3514370-16$15.00/0.
Figures



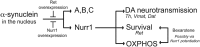
Similar articles
-
Retinoid X Receptor as a Therapeutic Target to Treat Neurological Disorders Associated with α-Synucleinopathy.Cells. 2025 May 9;14(10):685. doi: 10.3390/cells14100685. Cells. 2025. PMID: 40422188 Free PMC article.
-
α-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons.Sci Transl Med. 2012 Dec 5;4(163):163ra156. doi: 10.1126/scitranslmed.3004676. Sci Transl Med. 2012. PMID: 23220632
-
Bexarotene-Activated Retinoid X Receptors Regulate Neuronal Differentiation and Dendritic Complexity.J Neurosci. 2015 Aug 26;35(34):11862-76. doi: 10.1523/JNEUROSCI.1001-15.2015. J Neurosci. 2015. PMID: 26311769 Free PMC article.
-
Nurr1, an orphan nuclear receptor with essential functions in developing dopamine cells.Cell Tissue Res. 2004 Oct;318(1):45-52. doi: 10.1007/s00441-004-0974-7. Epub 2004 Sep 1. Cell Tissue Res. 2004. PMID: 15340833 Review.
-
NURR1 in Parkinson disease--from pathogenesis to therapeutic potential.Nat Rev Neurol. 2013 Nov;9(11):629-36. doi: 10.1038/nrneurol.2013.209. Epub 2013 Oct 15. Nat Rev Neurol. 2013. PMID: 24126627 Review.
Cited by
-
GDNF Therapy: Can We Make It Work?J Parkinsons Dis. 2021;11(3):1019-1022. doi: 10.3233/JPD-212706. J Parkinsons Dis. 2021. PMID: 33935108 Free PMC article.
-
α-Synuclein Translocates to the Nucleus to Activate Retinoic-Acid-Dependent Gene Transcription.iScience. 2020 Mar 27;23(3):100910. doi: 10.1016/j.isci.2020.100910. Epub 2020 Feb 14. iScience. 2020. PMID: 32120069 Free PMC article.
-
Impact of Pitx3 gene knockdown on glial cell line-derived neurotrophic factor transcriptional activity in dopaminergic neurons.Neural Regen Res. 2017 Aug;12(8):1347-1351. doi: 10.4103/1673-5374.213557. Neural Regen Res. 2017. PMID: 28966651 Free PMC article.
-
Nuclear Receptors as Therapeutic Targets for Neurodegenerative Diseases: Lost in Translation.Annu Rev Pharmacol Toxicol. 2019 Jan 6;59:237-261. doi: 10.1146/annurev-pharmtox-010818-021807. Epub 2018 Sep 12. Annu Rev Pharmacol Toxicol. 2019. PMID: 30208281 Free PMC article. Review.
-
Nuclear receptor 4A2 (NR4A2) is a druggable target for glioblastomas.J Neurooncol. 2020 Jan;146(1):25-39. doi: 10.1007/s11060-019-03349-y. Epub 2019 Nov 21. J Neurooncol. 2020. PMID: 31754919 Free PMC article.
References
-
- Anichtchik O, Calo L, Spillantini MG. Synaptic dysfunction in synucleinopathies. CNS Neurol Disord Drug Targets. 2013;12:1094–1100. - PubMed
-
- Barceló-Coblijn G, Golovko MY, Weinhofer I, Berger J, Murphy EJ. Brain neutral lipids mass is increased in alpha-synuclein gene-ablated mice. J Neurochem. 2007;101:132–141. - PubMed
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
