A Critical Gating Switch at a Modulatory Site in Neuronal Kir3 Channels
- PMID: 26490875
- PMCID: PMC4683693
- DOI: 10.1523/JNEUROSCI.1415-15.2015
A Critical Gating Switch at a Modulatory Site in Neuronal Kir3 Channels
Abstract
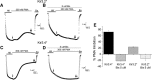
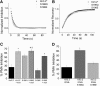
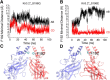
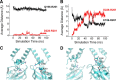
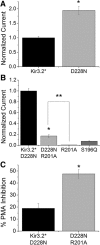
Inwardly rectifying potassium channels enforce tight control of resting membrane potential in excitable cells. The Kir3.2 channel, a member of the Kir3 subfamily of G-protein-activated potassium channels (GIRKs), plays several roles in the nervous system, including key responsibility in the GABAB pathway of inhibition, in pain perception pathways via opioid receptors, and is also involved in alcoholism. PKC phosphorylation acts on the channel to reduce activity, yet the mechanism is incompletely understood. Using the heterologous Xenopus oocyte system combined with molecular dynamics simulations, we show that PKC modulation of channel activity is dependent on Ser-196 in Kir3.2 such that, when this site is phosphorylated, the channel is less sensitive to PKC inhibition. This reduced inhibition is dependent on an interaction between phospho-Ser (SEP)-196 and Arg-201, reducing Arg-201 interaction with the sodium-binding site Asp-228. Neutralization of either SEP-196 or Arg-201 leads to a channel with reduced activity and increased sensitivity to PKC inhibition. This study clarifies the role of Ser-196 as an allosteric modulator of PKC inhibition and suggests that the SEP-196/Arg-201 interaction is critical for maintaining maximal channel activity.
Significance statement: The inwardly rectifying potassium 3.2 (Kir3.2) channel is found principally in neurons that regulate diverse brain functions, including pain perception, alcoholism, and substance addiction. Activation or inhibition of this channel leads to changes in neuronal firing and chemical message transmission. The Kir3.2 channel is subject to regulation by intracellular signals including sodium, G-proteins, ethanol, the phospholipid phosphatidylinositol bis-phosphate, and phosphorylation by protein kinases. Here, we take advantage of the recently published structure of Kir3.2 to provide an in-depth molecular view of how phosphorylation of a specific residue previously thought to be the target of PKC promotes channel gating and acts as an allosteric modulator of PKC-mediated inhibition.
Keywords: GIRK; Kir3; PIP2; PKC.
Copyright © 2015 the authors 0270-6474/15/3514397-09$15.00/0.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
