Reversible Deactivation of Motor Cortex Reveals Functional Connectivity with Posterior Parietal Cortex in the Prosimian Galago (Otolemur garnettii)
- PMID: 26490876
- PMCID: PMC4683694
- DOI: 10.1523/JNEUROSCI.1468-15.2015
Reversible Deactivation of Motor Cortex Reveals Functional Connectivity with Posterior Parietal Cortex in the Prosimian Galago (Otolemur garnettii)
Abstract
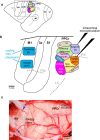
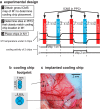
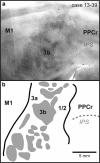
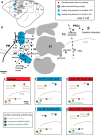
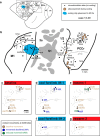
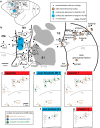
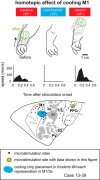
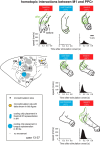
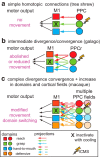
We examined the functional macrocircuitry of frontoparietal networks in the neocortex of prosimian primates (Otolemur garnettii) using a microfluidic thermal regulator to reversibly deactivate selected regions of motor cortex (M1). During deactivation of either forelimb or mouth/face movement domains within M1, we used long-train intracortical microstimulation techniques to evoke movements from the rostral division of posterior parietal cortex (PPCr). We found that deactivation of M1 movement domains in most instances abolished movements evoked in PPCr. The most common effect of deactivating M1 was to abolish evoked movements in a homotopic domain in PPCr. For example, deactivating M1 forelimb lift domains resulted in loss of evoked movement in forelimb domains in PPCr. However, at some sites, we also observed heterotopic effects; deactivating a specific domain in M1 (e.g., forelimb lift) resulted in loss of evoked movement in a different movement domain in PPCr (e.g., hand-to-mouth or eye-blink). At most sites examined in PPCr, rewarming M1 resulted in a reestablishment of the baseline movement at the same amplitude as that observed before cooling. However, at some sites, reactivation did not result in a return to baseline movement or to the full amplitude of the baseline movement. We discuss our findings in the context of frontoparietal circuits and how they may subserve a repertoire of ecologically relevant behaviors.
Significance statement: The posterior parietal cortex (PPC) of primates integrates sensory information used to guide movements. Different modules within PPC and motor cortex (M1) appear to control various motor behaviors (e.g., reaching, defense, and feeding). How these modules work together may vary across species and may explain differences in dexterity and even the capacity for tool use. We investigated the functional connectivity of these modules in galagos, a prosimian primate with relatively simple frontoparietal circuitry. By deactivating a reaching module in M1, we interfered with the function of similar PPC modules and occasionally unrelated PPC modules as well (e.g., eye blink). This circuitry in galagos, therefore, is more complex than in nonprimates, indicating that it has been altered with the expansion of primate PPC.
Keywords: cooling inactivation; lesion; muscimol; network; primate; reaching.
Copyright © 2015 the authors 0270-6474/15/3514406-17$15.00/0.
Figures



Similar articles
-
The dorsal stream of visual processing and action-specific domains in parietal and frontal cortex in primates.J Comp Neurol. 2023 Dec;531(18):1897-1908. doi: 10.1002/cne.25489. Epub 2023 Apr 28. J Comp Neurol. 2023. PMID: 37118872 Free PMC article. Review.
-
Effects of muscimol inactivations of functional domains in motor, premotor, and posterior parietal cortex on complex movements evoked by electrical stimulation.J Neurophysiol. 2014 Mar;111(5):1100-19. doi: 10.1152/jn.00491.2013. Epub 2013 Dec 18. J Neurophysiol. 2014. PMID: 24353298 Free PMC article.
-
Interactions within and between parallel parietal-frontal networks involved in complex motor behaviors in prosimian galagos and a squirrel monkey.J Neurophysiol. 2020 Jan 1;123(1):34-56. doi: 10.1152/jn.00576.2019. Epub 2019 Nov 6. J Neurophysiol. 2020. PMID: 31693452 Free PMC article.
-
Reversible deactivation of motor cortex reveals that areas in parietal cortex are differentially dependent on motor cortex for the generation of movement.J Neurophysiol. 2024 Jan 1;131(1):106-123. doi: 10.1152/jn.00086.2023. Epub 2023 Dec 13. J Neurophysiol. 2024. PMID: 38092416 Free PMC article.
-
Evolution of posterior parietal cortex and parietal-frontal networks for specific actions in primates.J Comp Neurol. 2016 Feb 15;524(3):595-608. doi: 10.1002/cne.23838. Epub 2015 Jul 21. J Comp Neurol. 2016. PMID: 26101180 Free PMC article. Review.
Cited by
-
The Evolution of the Pulvinar Complex in Primates and Its Role in the Dorsal and Ventral Streams of Cortical Processing.Vision (Basel). 2019 Dec 30;4(1):3. doi: 10.3390/vision4010003. Vision (Basel). 2019. PMID: 31905909 Free PMC article. Review.
-
The dorsal stream of visual processing and action-specific domains in parietal and frontal cortex in primates.J Comp Neurol. 2023 Dec;531(18):1897-1908. doi: 10.1002/cne.25489. Epub 2023 Apr 28. J Comp Neurol. 2023. PMID: 37118872 Free PMC article. Review.
-
Thalamic connections of the caudal part of the posterior parietal cortex differ from the rostral part in galagos (Otolemur garnettii).J Comp Neurol. 2023 Dec;531(17):1752-1771. doi: 10.1002/cne.25537. Epub 2023 Sep 13. J Comp Neurol. 2023. PMID: 37702312 Free PMC article.
-
Cortical connections of the functional domain for climbing or running in posterior parietal cortex of galagos.J Comp Neurol. 2021 Jul 1;529(10):2789-2812. doi: 10.1002/cne.25123. Epub 2021 Mar 4. J Comp Neurol. 2021. PMID: 33550608 Free PMC article.
-
Extensive complex neocortical movement topography devolves to simple output following experimental stroke in mice.Front Syst Neurosci. 2023 Jun 7;17:1162664. doi: 10.3389/fnsys.2023.1162664. eCollection 2023. Front Syst Neurosci. 2023. PMID: 37350800 Free PMC article.
References
-
- Baldwin MK, Cooke DF, Gordon A, Krubitzer L. Revealing functional organization of frontoparietal networks in tree shrews (Tupaia belangeri) using reversible inactivation. Soc Neurosci Abstr. 2014;40:446.02.
