A non-canonical multisubunit RNA polymerase encoded by a giant bacteriophage
- PMID: 26490960
- PMCID: PMC4666361
- DOI: 10.1093/nar/gkv1095
A non-canonical multisubunit RNA polymerase encoded by a giant bacteriophage
Abstract
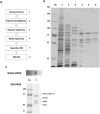
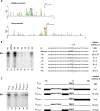
The infection of Pseudomonas aeruginosa by the giant bacteriophage phiKZ is resistant to host RNA polymerase (RNAP) inhibitor rifampicin. phiKZ encodes two sets of polypeptides that are distantly related to fragments of the two largest subunits of cellular multisubunit RNAPs. Polypeptides of one set are encoded by middle phage genes and are found in the phiKZ virions. Polypeptides of the second set are encoded by early phage genes and are absent from virions. Here, we report isolation of a five-subunit RNAP from phiKZ-infected cells. Four subunits of this enzyme are cellular RNAP subunits homologs of the non-virion set; the fifth subunit is a protein of unknown function. In vitro, this complex initiates transcription from late phiKZ promoters in rifampicin-resistant manner. Thus, this enzyme is a non-virion phiKZ RNAP responsible for transcription of late phage genes. The phiKZ RNAP lacks identifiable assembly and promoter specificity subunits/factors characteristic for eukaryal, archaeal and bacterial RNAPs and thus provides a unique model for comparative analysis of the mechanism, regulation and evolution of this important class of enzymes.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Cheetham G.M., Steitz T.A. Structure of a transcribing T7 RNA polymerase initiation complex. Science. 1999;286:2305–2309. - PubMed
-
- Cermakian N., Ikeda T.M., Miramontes P., Lang B.F., Gray M.W., Cedergren R. On the evolution of the single-subunit RNA polymerases. J. Mol. Evol. 1997;45:671–681. - PubMed
-
- Zaychikov E., Martin E., Denissova L., Kozlov M., Markovtsov V., Kashlev M., Heumann H., Nikiforov V., Goldfarb A., Mustaev A. Mapping of catalytic residues in the RNA polymerase active center. Science. 1996;273:107–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

