Lactate Contributes to Glyceroneogenesis and Glyconeogenesis in Skeletal Muscle by Reversal of Pyruvate Kinase
- PMID: 26491014
- PMCID: PMC4683270
- DOI: 10.1074/jbc.M115.689174
Lactate Contributes to Glyceroneogenesis and Glyconeogenesis in Skeletal Muscle by Reversal of Pyruvate Kinase
Abstract
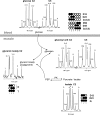
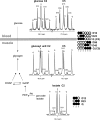
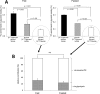
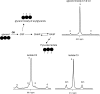
Phosphoenolpyruvate (PEP) generated from pyruvate is required for de novo synthesis of glycerol and glycogen in skeletal muscle. One possible pathway involves synthesis of PEP from the citric acid cycle intermediates via PEP carboxykinase, whereas another could involve reversal of pyruvate kinase (PK). Earlier studies have reported that reverse flux through PK can contribute carbon precursors for glycogen synthesis in muscle, but the physiological importance of this pathway remains uncertain especially in the setting of high plasma glucose. In addition, although PEP is a common intermediate for both glyconeogenesis and glyceroneogenesis, the importance of reverse PK in de novo glycerol synthesis has not been examined. Here we studied the contribution of reverse PK to synthesis of glycogen and the glycerol moiety of acylglycerols in skeletal muscle of animals with high plasma glucose. Rats received a single intraperitoneal bolus of glucose, glycerol, and lactate under a fed or fasted state. Only one of the three substrates was (13)C-labeled in each experiment. After 3 h of normal awake activity, the animals were sacrificed, and the contribution from each substrate to glycogen and the glycerol moiety of acylglycerols was evaluated. The fraction of (13)C labeling in glycogen and the glycerol moiety exceeded the possible contribution from either plasma glucose or muscle oxaloacetate. The reverse PK served as a common route for both glyconeogenesis and glyceroneogenesis in the skeletal muscle of rats with high plasma glucose. The activity of pyruvate carboxylase was low in muscle, and no PEP carboxykinase activity was detected.
Keywords: citric acid cycle; glycerol; glycobiology; glycogen synthase; phosphoenolpyruvate carboxykinase; pyruvate carboxylase (PC); pyruvate dehydrogenase complex (PDC); pyruvate kinase; skeletal muscle metabolism.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
Evidence for reverse flux through pyruvate kinase in skeletal muscle.Am J Physiol Endocrinol Metab. 2009 Apr;296(4):E748-57. doi: 10.1152/ajpendo.90935.2008. Epub 2009 Feb 3. Am J Physiol Endocrinol Metab. 2009. PMID: 19190256 Free PMC article.
-
Metabolism of glycerol, glucose, and lactate in the citric acid cycle prior to incorporation into hepatic acylglycerols.J Biol Chem. 2013 May 17;288(20):14488-14496. doi: 10.1074/jbc.M113.461947. Epub 2013 Apr 9. J Biol Chem. 2013. PMID: 23572519 Free PMC article.
-
Glyconeogenic pathway in isolated skeletal muscles of rats.Can J Physiol Pharmacol. 2002 Feb;80(2):164-9. doi: 10.1139/y02-013. Can J Physiol Pharmacol. 2002. PMID: 11934259
-
Quantitative assessment of pathways for lactate disposal in skeletal muscle fiber types.Med Sci Sports Exerc. 2000 Apr;32(4):772-7. doi: 10.1097/00005768-200004000-00009. Med Sci Sports Exerc. 2000. PMID: 10776896 Review.
-
Glucose and lactate metabolism by Actinomyces naeslundii.Crit Rev Oral Biol Med. 1999;10(4):487-503. doi: 10.1177/10454411990100040501. Crit Rev Oral Biol Med. 1999. PMID: 10634585 Review.
Cited by
-
Utilization of lactic acid in human myotubes and interplay with glucose and fatty acid metabolism.Sci Rep. 2018 Jun 29;8(1):9814. doi: 10.1038/s41598-018-28249-5. Sci Rep. 2018. PMID: 29959350 Free PMC article.
-
Pyruvate Dehydrogenase Complex in Neonatal Hypoxic-Ischemic Brain Injury.ACS Pharmacol Transl Sci. 2023 Dec 22;7(1):42-47. doi: 10.1021/acsptsci.3c00191. eCollection 2024 Jan 12. ACS Pharmacol Transl Sci. 2023. PMID: 38230287 Free PMC article. Review.
-
Effect of post-exercise lactate administration on glycogen repletion and signaling activation in different types of mouse skeletal muscle.Curr Res Physiol. 2020 Jul 30;3:34-43. doi: 10.1016/j.crphys.2020.07.002. eCollection 2020 Dec. Curr Res Physiol. 2020. PMID: 34746818 Free PMC article.
-
Tracking the carbons supplying gluconeogenesis.J Biol Chem. 2020 Oct 16;295(42):14419-14429. doi: 10.1074/jbc.REV120.012758. Epub 2020 Aug 13. J Biol Chem. 2020. PMID: 32817317 Free PMC article. Review.
-
Lactate metabolism in human health and disease.Signal Transduct Target Ther. 2022 Sep 1;7(1):305. doi: 10.1038/s41392-022-01151-3. Signal Transduct Target Ther. 2022. PMID: 36050306 Free PMC article. Review.
References
-
- McLane J. A., and Holloszy J. O. (1979) Glycogen synthesis from lactate in the three types of skeletal muscle. J. Biol. Chem. 254, 6548–6553 - PubMed
-
- Bonen A., McDermott J. C., and Tan M. H. (1990) Glycogenesis and glyconeogenesis in skeletal muscle: effects of pH and hormones. Am. J. Physiol. 258, E693–E700 - PubMed
-
- Dobson G. P., Hitchins S., and Teague W. E. Jr. (2002) Thermodynamics of the pyruvate kinase reaction and the reversal of glycolysis in heart and skeletal muscle. J. Biol. Chem. 277, 27176–27182 - PubMed
-
- Connett R. J. (1979) Glyconeogenesis from lactate in frog striated muscle. Am. J. Physiol. 237, C231–C236 - PubMed
-
- McQuate J. T., and Utter M. F. (1959) Equilibrium and kinetic studies of the pyruvic kinase reaction. J. Biol. Chem. 234, 2151–2157 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41 RR002584/RR/NCRR NIH HHS/United States
- DK58398/DK/NIDDK NIH HHS/United States
- HL34557/HL/NHLBI NIH HHS/United States
- EB 015908/EB/NIBIB NIH HHS/United States
- R01 HL034557/HL/NHLBI NIH HHS/United States
- P01 DK058398/DK/NIDDK NIH HHS/United States
- RR 002584/RR/NCRR NIH HHS/United States
- DK099289/DK/NIDDK NIH HHS/United States
- DK078933/DK/NIDDK NIH HHS/United States
- P41 EB015908/EB/NIBIB NIH HHS/United States
- R37 HL034557/HL/NHLBI NIH HHS/United States
- R01 DK099289/DK/NIDDK NIH HHS/United States
- K01 DK078933/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

