Defensive Perimeter in the Central Nervous System: Predominance of Astrocytes and Astrogliosis during Recovery from Varicella-Zoster Virus Encephalitis
- PMID: 26491149
- PMCID: PMC4702565
- DOI: 10.1128/JVI.02389-15
Defensive Perimeter in the Central Nervous System: Predominance of Astrocytes and Astrogliosis during Recovery from Varicella-Zoster Virus Encephalitis
Abstract
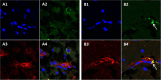
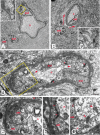
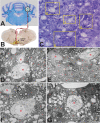
Varicella-zoster virus (VZV) is a highly neurotropic virus that can cause infections in both the peripheral nervous system and the central nervous system. Several studies of VZV reactivation in the peripheral nervous system (herpes zoster) have been published, while exceedingly few investigations have been carried out in a human brain. Notably, there is no animal model for VZV infection of the central nervous system. In this report, we characterized the cellular environment in the temporal lobe of a human subject who recovered from focal VZV encephalitis. The approach included not only VZV DNA/RNA analyses but also a delineation of infected cell types (neurons, microglia, oligodendrocytes, and astrocytes). The average VZV genome copy number per cell was 5. Several VZV regulatory and structural gene transcripts and products were detected. When colocalization studies were performed to determine which cell types harbored the viral proteins, the majority of infected cells were astrocytes, including aggregates of astrocytes. Evidence of syncytium formation within the aggregates included the continuity of cytoplasm positive for the VZV glycoprotein H (gH) fusion-complex protein within a cellular profile with as many as 80 distinct nuclei. As with other causes of brain injury, these results suggested that astrocytes likely formed a defensive perimeter around foci of VZV infection (astrogliosis). Because of the rarity of brain samples from living humans with VZV encephalitis, we compared our VZV results with those found in a rat encephalitis model following infection with the closely related pseudorabies virus and observed similar perimeters of gliosis.
Importance: Investigations of VZV-infected human brain from living immunocompetent human subjects are exceedingly rare. Therefore, much of our knowledge of VZV neuropathogenesis is gained from studies of VZV-infected brains obtained at autopsy from immunocompromised patients. These are not optimal samples with which to investigate a response by a human host to VZV infection. In this report, we examined both flash-frozen and paraffin-embedded formalin-fixed brain tissue of an otherwise healthy young male with focal VZV encephalitis, most likely acquired from VZV reactivation in the trigeminal ganglion. Of note, the cellular response to VZV infection mimicked the response to other causes of trauma to the brain, namely, an ingress of astrocytes and astrogliosis around an infectious focus. Many of the astrocytes themselves were infected; astrocytes aggregated in clusters. We postulate that astrogliosis represents a successful defense mechanism by an immunocompetent human host to eliminate VZV reactivation within neurons.
Copyright © 2015 Carpenter et al.
Figures






Similar articles
-
Determinants of neurological syndromes caused by varicella zoster virus (VZV).J Neurovirol. 2020 Aug;26(4):482-495. doi: 10.1007/s13365-020-00857-w. Epub 2020 Jun 3. J Neurovirol. 2020. PMID: 32495195 Free PMC article. Review.
-
Focal encephalitis following varicella-zoster virus reactivation without rash in a healthy immunized young adult.J Infect Dis. 2014 Sep 1;210(5):713-6. doi: 10.1093/infdis/jiu137. Epub 2014 Mar 5. J Infect Dis. 2014. PMID: 24604820 Free PMC article.
-
Dysregulated Glycoprotein B-Mediated Cell-Cell Fusion Disrupts Varicella-Zoster Virus and Host Gene Transcription during Infection.J Virol. 2016 Dec 16;91(1):e01613-16. doi: 10.1128/JVI.01613-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795423 Free PMC article.
-
Presence, distribution and spread of productive varicella zoster virus infection in nervous tissues.Brain. 1992 Apr;115 ( Pt 2):383-98. doi: 10.1093/brain/115.2.383. Brain. 1992. PMID: 1318768
-
Immune evasion as a pathogenic mechanism of varicella zoster virus.Semin Immunol. 2001 Feb;13(1):27-39. doi: 10.1006/smim.2001.0293. Semin Immunol. 2001. PMID: 11289797 Review.
Cited by
-
Zika Fetal Neuropathogenesis: Etiology of a Viral Syndrome.PLoS Negl Trop Dis. 2016 Aug 25;10(8):e0004877. doi: 10.1371/journal.pntd.0004877. eCollection 2016 Aug. PLoS Negl Trop Dis. 2016. PMID: 27560129 Free PMC article. Review.
-
Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).J Transl Med. 2018 Oct 1;16(1):268. doi: 10.1186/s12967-018-1644-y. J Transl Med. 2018. PMID: 30285773 Free PMC article. Review.
-
Development of a skin- and neuro-attenuated live vaccine for varicella.Nat Commun. 2022 Feb 11;13(1):824. doi: 10.1038/s41467-022-28329-1. Nat Commun. 2022. PMID: 35149692 Free PMC article.
-
The round trip model for severe herpes zoster caused by live attenuated varicella vaccine virus.J Med Virol. 2020 Aug;92(8):938-940. doi: 10.1002/jmv.25664. Epub 2020 Jan 17. J Med Virol. 2020. PMID: 31943220 Free PMC article.
-
Varicella zoster virus differentially alters morphology and suppresses proinflammatory cytokines in primary human spinal cord and hippocampal astrocytes.J Neuroinflammation. 2018 Nov 15;15(1):318. doi: 10.1186/s12974-018-1360-9. J Neuroinflammation. 2018. PMID: 30442152 Free PMC article.
References
-
- Weller TH. 1983. Varicella and herpes zoster. Changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med 309:1434–1440. - PubMed
-
- Arvin AM. 2006. Investigations of the pathogenesis of varicella zoster virus infection in the SCIDhu mouse model. Herpes 13:75–80. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

