A Single Maturation Cleavage Site in Adenovirus Impacts Cell Entry and Capsid Assembly
- PMID: 26491163
- PMCID: PMC4702569
- DOI: 10.1128/JVI.02014-15
A Single Maturation Cleavage Site in Adenovirus Impacts Cell Entry and Capsid Assembly
Abstract
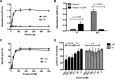
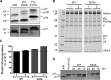
Proteolytic maturation drives the conversion of stable, immature virus particles to a mature, metastable state primed for cell infection. In the case of human adenovirus, this proteolytic cleavage is mediated by the virally encoded protease AVP. Protein VI, an internal capsid cement protein and substrate for AVP, is cleaved at two sites, one of which is near the N terminus of the protein. In mature capsids, the 33 residues at the N terminus of protein VI (pVIn) are sequestered inside the cavity formed by peripentonal hexon trimers at the 5-fold vertex. Here, we describe a glycine-to-alanine mutation in the N-terminal cleavage site of protein VI that profoundly impacts proteolytic processing, the generation of infectious particles, and cell entry. The phenotypic effects associated with this mutant provide a mechanistic framework for understanding the multifunctional nature of protein VI. Based on our findings, we propose that the primary function of the pVIn peptide is to mediate interactions between protein VI and hexon during virus replication, driving hexon nuclear accumulation and particle assembly. Once particles are assembled, AVP-mediated cleavage facilitates the release of the membrane lytic region at the amino terminus of mature VI, allowing it to lyse the endosome during cell infection. These findings highlight the importance of a single maturation cleavage site for both infectious particle production and cell entry and emphasize the exquisite spatiotemporal regulation governing adenovirus assembly and disassembly.
Importance: Postassembly virus maturation is a cornerstone principle in virology. However, a mechanistic understanding of how icosahedral viruses utilize this process to transform immature capsids into infection-competent particles is largely lacking. Adenovirus maturation involves proteolytic processing of seven precursor proteins. There is currently no information for the role of each independent cleavage event in the generation of infectious virions. To address this, we investigated the proteolytic maturation of one adenovirus precursor molecule, protein VI. Structurally, protein VI cements the outer capsid shell and links it to the viral core. Functionally, protein VI is involved in endosome disruption, subcellular trafficking, transcription activation, and virus assembly. Our studies demonstrate that the multifunctional nature of protein VI is largely linked to its maturation. Through mutational analysis, we show that disrupting the N-terminal cleavage of preprotein VI has major deleterious effects on the assembly of infectious virions and their subsequent ability to infect host cells.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Structures and organization of adenovirus cement proteins provide insights into the role of capsid maturation in virus entry and infection.Proc Natl Acad Sci U S A. 2014 Aug 12;111(32):11715-20. doi: 10.1073/pnas.1408462111. Epub 2014 Jul 28. Proc Natl Acad Sci U S A. 2014. PMID: 25071205 Free PMC article.
-
The cleaved N-terminus of pVI binds peripentonal hexons in mature adenovirus.J Mol Biol. 2014 May 1;426(9):1971-9. doi: 10.1016/j.jmb.2014.02.022. Epub 2014 Mar 5. J Mol Biol. 2014. PMID: 24613303 Free PMC article.
-
Dynamic competition for hexon binding between core protein VII and lytic protein VI promotes adenovirus maturation and entry.Proc Natl Acad Sci U S A. 2020 Jun 16;117(24):13699-13707. doi: 10.1073/pnas.1920896117. Epub 2020 May 28. Proc Natl Acad Sci U S A. 2020. PMID: 32467158 Free PMC article.
-
Structure, function and dynamics in adenovirus maturation.Viruses. 2014 Nov 21;6(11):4536-70. doi: 10.3390/v6114536. Viruses. 2014. PMID: 25421887 Free PMC article. Review.
-
Understanding Post Entry Sorting of Adenovirus Capsids; A Chance to Change Vaccine Vector Properties.Viruses. 2021 Jun 24;13(7):1221. doi: 10.3390/v13071221. Viruses. 2021. PMID: 34202573 Free PMC article. Review.
Cited by
-
RETRACTED: Role of Protein VII in the Production of Infectious Bovine Adenovirus-3 Virion.Viruses. 2024 Aug 19;16(8):1323. doi: 10.3390/v16081323. Viruses. 2024. Retraction in: Viruses. 2025 Aug 19;17(8):1133. doi: 10.3390/v17081133. PMID: 39205297 Free PMC article. Retracted.
-
Insights into Adenovirus Uncoating from Interactions with Integrins and Mediators of Host Immunity.Viruses. 2016 Dec 21;8(12):337. doi: 10.3390/v8120337. Viruses. 2016. PMID: 28009821 Free PMC article. Review.
-
Proteolytic Cleavage of Bovine Adenovirus 3-Encoded pVIII.J Virol. 2017 Apr 28;91(10):e00211-17. doi: 10.1128/JVI.00211-17. Print 2017 May 15. J Virol. 2017. PMID: 28298598 Free PMC article.
-
A single point mutation in precursor protein VI doubles the mechanical strength of human adenovirus.J Biol Phys. 2018 Jun;44(2):119-132. doi: 10.1007/s10867-017-9479-y. Epub 2017 Dec 15. J Biol Phys. 2018. PMID: 29243050 Free PMC article.
-
Disparate Entry of Adenoviruses Dictates Differential Innate Immune Responses on the Ocular Surface.Microorganisms. 2019 Sep 13;7(9):351. doi: 10.3390/microorganisms7090351. Microorganisms. 2019. PMID: 31540200 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

