Targeting Swine Leukocyte Antigen Class I Molecules for Proteasomal Degradation by the nsp1α Replicase Protein of the Chinese Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus Strain JXwn06
- PMID: 26491168
- PMCID: PMC4702659
- DOI: 10.1128/JVI.02307-15
Targeting Swine Leukocyte Antigen Class I Molecules for Proteasomal Degradation by the nsp1α Replicase Protein of the Chinese Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus Strain JXwn06
Abstract
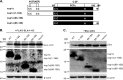
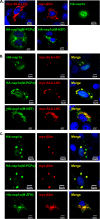
Porcine reproductive and respiratory syndrome virus (PRRSV) is a critical pathogen of swine, and infections by this virus often result in delayed, low-level induction of cytotoxic T lymphocyte (CTL) responses in pigs. Here, we report that a Chinese highly pathogenic PRRSV strain possessed the ability to downregulate swine leukocyte antigen class I (SLA-I) molecules on the cell surface of porcine alveolar macrophages and target them for degradation in a manner that was dependent on the ubiquitin-proteasome system. Moreover, we found that the nsp1α replicase protein contributed to this property of PRRSV. Further mutagenesis analyses revealed that this function of nsp1α required the intact molecule, including the zinc finger domain, but not the cysteine protease activity. More importantly, we found that nsp1α was able to interact with both chains of SLA-I, a requirement that is commonly needed for many viral proteins to target their cellular substrates for proteasomal degradation. Together, our findings provide critical insights into the mechanisms of how PRRSV might evade cellular immunity and also add a new role for nsp1α in PRRSV infection.
Importance: PRRSV infections often result in delayed, low-level induction of CTL responses in pigs. Deregulation of this immunity is thought to prevent the virus from clearance in an efficient and timely manner, contributing to persistent infections in swineherds. Our studies in this report provide critical insight into the mechanism of how PRRSV might evade CTL responses. In addition, our findings add a new role for nsp1α, a critical viral factor involved in antagonizing host innate immunity.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Nonstructural Protein 4 of Porcine Reproductive and Respiratory Syndrome Virus Modulates Cell Surface Swine Leukocyte Antigen Class I Expression by Downregulating β2-Microglobulin Transcription.J Virol. 2017 Feb 14;91(5):e01755-16. doi: 10.1128/JVI.01755-16. Print 2017 Mar 1. J Virol. 2017. PMID: 28003480 Free PMC article.
-
Porcine Reproductive and Respiratory Syndrome Virus Antagonizes JAK/STAT3 Signaling via nsp5, Which Induces STAT3 Degradation.J Virol. 2017 Jan 18;91(3):e02087-16. doi: 10.1128/JVI.02087-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27881658 Free PMC article.
-
Mapping the Key Residues within the Porcine Reproductive and Respiratory Syndrome Virus nsp1α Replicase Protein Required for Degradation of Swine Leukocyte Antigen Class I Molecules.Viruses. 2022 Mar 26;14(4):690. doi: 10.3390/v14040690. Viruses. 2022. PMID: 35458420 Free PMC article.
-
The PRRSV replicase: exploring the multifunctionality of an intriguing set of nonstructural proteins.Virus Res. 2010 Dec;154(1-2):61-76. doi: 10.1016/j.virusres.2010.07.030. Epub 2010 Aug 7. Virus Res. 2010. PMID: 20696193 Free PMC article. Review.
-
Regulation and evasion of antiviral immune responses by porcine reproductive and respiratory syndrome virus.Virus Res. 2015 Apr 16;202:101-11. doi: 10.1016/j.virusres.2014.12.014. Epub 2014 Dec 18. Virus Res. 2015. PMID: 25529442 Free PMC article. Review.
Cited by
-
Transcriptome Analysis Reveals Dynamic Gene Expression Profiles in Porcine Alveolar Macrophages in Response to the Chinese Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus.Biomed Res Int. 2018 Apr 29;2018:1538127. doi: 10.1155/2018/1538127. eCollection 2018. Biomed Res Int. 2018. PMID: 29854728 Free PMC article.
-
Reduced Virus Load in Lungs of Pigs Challenged with Porcine Reproductive and Respiratory Syndrome Virus after Vaccination with Virus Replicon Particles Encoding Conserved PRRSV Cytotoxic T-Cell Epitopes.Vaccines (Basel). 2021 Mar 2;9(3):208. doi: 10.3390/vaccines9030208. Vaccines (Basel). 2021. PMID: 33801369 Free PMC article.
-
The S Gene Is Necessary but Not Sufficient for the Virulence of Porcine Epidemic Diarrhea Virus Novel Variant Strain BJ2011C.J Virol. 2018 Jun 13;92(13):e00603-18. doi: 10.1128/JVI.00603-18. Print 2018 Jul 1. J Virol. 2018. PMID: 29695430 Free PMC article.
-
Role of microRNAs in host defense against porcine reproductive and respiratory syndrome virus infection: a hidden front line.Front Immunol. 2024 Mar 25;15:1376958. doi: 10.3389/fimmu.2024.1376958. eCollection 2024. Front Immunol. 2024. PMID: 38590524 Free PMC article. Review.
-
Molecular and Cellular Mechanisms for PRRSV Pathogenesis and Host Response to Infection.Virus Res. 2020 Sep;286:197980. doi: 10.1016/j.virusres.2020.197980. Epub 2020 Apr 18. Virus Res. 2020. PMID: 32311386 Free PMC article. Review.
References
-
- Cavanagh D. 1997. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol 142:629–633. - PubMed
-
- Garner MG, Whan IF, Gard GP, Phillips D. 2001. The expected economic impact of selected exotic diseases on the pig industry of Australia. Rev Sci Tech 20:671–685. - PubMed
-
- Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ. 2005. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc 227:385–392. doi:10.2460/javma.2005.227.385. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

