Necroptotic Cell Death Signaling and Execution Pathway: Lessons from Knockout Mice
- PMID: 26491219
- PMCID: PMC4600508
- DOI: 10.1155/2015/128076
Necroptotic Cell Death Signaling and Execution Pathway: Lessons from Knockout Mice
Abstract
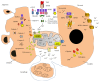
Under stress conditions, cells in living tissue die by apoptosis or necrosis depending on the activation of the key molecules within a dying cell that either transduce cell survival or death signals that actively destroy the sentenced cell. Multiple extracellular (pH, heat, oxidants, and detergents) or intracellular (DNA damage and Ca(2+) overload) stress conditions trigger various types of the nuclear, endoplasmic reticulum (ER), cytoplasmatic, and mitochondrion-centered signaling events that allow cells to preserve the DNA integrity, protein folding, energetic, ionic and redox homeostasis, thus escaping from injury. Along the transition from reversible to irreversible injury, death signaling is highly heterogeneous and damaged cells may engage autophagy, apoptotic, or necrotic cell death programs. Studies on multiple double- and triple- knockout mice identified caspase-8, flip, and fadd genes as key regulators of embryonic lethality and inflammation. Caspase-8 has a critical role in pro- and antinecrotic signaling pathways leading to the activation of receptor interacting protein kinase 1 (RIPK1), RIPK3, and the mixed kinase domain-like (MLKL) for a convergent execution pathway of necroptosis or regulated necrosis. Here we outline the recent discoveries into how the necrotic cell death execution pathway is engaged in many physiological and pathological outcome based on genetic analysis of knockout mice.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

