Systematic review and meta-analysis: bezafibrate in patients with primary biliary cirrhosis
- PMID: 26491252
- PMCID: PMC4599574
- DOI: 10.2147/DDDT.S92041
Systematic review and meta-analysis: bezafibrate in patients with primary biliary cirrhosis
Erratum in
-
Systematic review and meta-analysis: bezafibrate in patients with primary biliary cirrhosis [Erratum].Drug Des Devel Ther. 2015 Nov 6;9:5947. doi: 10.2147/DDDT.S98298. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26604692 Free PMC article. No abstract available.
Abstract
Background and aim: Ursodeoxycholic acid (UDCA) is the standard treatment for primary biliary cirrhosis (PBC), but not all cases respond well. Evidence has shown that combination therapy of UDCA with bezafibrate significantly improved liver function. A meta-analysis was performed to assess the efficacy and safety of UDCA and bezafibrate combination therapy in the treatment of PBC.
Results: Nine trials, with a total of 269 patients, were included in the analysis. The bias risk of these trials was high. Compared with UDCA alone, the combination with bezafibrate improved the Mayo risk score (mean difference [MD], 0.60; 95% confidence interval [CI], 0.25-0.95; P=0.0008) and liver biochemistry: alkaline phosphatase (MD, -238.21 IU/L; 95% CI, -280.83 to -195.60; P<0.00001); gamma-glutamyltransferase (MD, -38.23 IU/L; 95% CI, -50.16 to -25.85; P<0.00001); immunoglobulin M (MD, -128.63 IU/L; 95% CI, -151.55 to -105.71; P<0.00001); bilirubin (MD, -0.20 mg/dL; 95% CI, -0.33 to -0.07; P=0.002); triglycerides (MD, -26.84 mg/dL; 95% CI, -36.51 to -17.17; P<0.0001); total cholesterol (MD, -21.58 mg/dL; 95% CI, -30.81 to -12.34; P<0.0001), and serum alanine aminotransferase (MD, -10.24 IU/L; 95% CI, -12.65 to -78.5; P<0.00001). However, combination therapy showed no significant differences in the incidence of all-cause mortality or pruritus, and may have resulted in more adverse events (risk ratio [RR], 0.22; 95% CI, 0.07-0.67; P=0.008).
Conclusion: Combination therapy improved liver biochemistry and the prognosis of PBC, but did not improve clinical symptoms or incidence of death. Attention should be paid to adverse events when using bezafibrate.
Keywords: bezafibrate; meta-analysis; primary biliary cirrhosis; ursodeoxycholic acid.
Figures



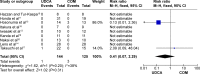











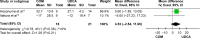
Similar articles
-
Bezafibrate for primary biliary cirrhosis.Cochrane Database Syst Rev. 2012 Jan 18;1(1):CD009145. doi: 10.1002/14651858.CD009145.pub2. Cochrane Database Syst Rev. 2012. PMID: 22259000 Free PMC article.
-
Ursodeoxycholic acid for primary biliary cirrhosis.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD000551. doi: 10.1002/14651858.CD000551.pub3. Cochrane Database Syst Rev. 2012. PMID: 23235576 Free PMC article.
-
Combination therapy of fenofibrate and ursodeoxycholic acid in patients with primary biliary cirrhosis who respond incompletely to UDCA monotherapy: a meta-analysis.Drug Des Devel Ther. 2015 May 25;9:2757-66. doi: 10.2147/DDDT.S79837. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26045661 Free PMC article.
-
Ursodeoxycholic acid for primary biliary cirrhosis.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD000551. doi: 10.1002/14651858.CD000551.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2012 Dec 12;12:CD000551. doi: 10.1002/14651858.CD000551.pub3. PMID: 18677775 Updated.
-
Ursodeoxycholic acid for primary biliary cirrhosis.Cochrane Database Syst Rev. 2002;(1):CD000551. doi: 10.1002/14651858.CD000551. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2008 Jul 16;(3):CD000551. doi: 10.1002/14651858.CD000551.pub2. PMID: 11869580 Updated.
Cited by
-
Efficacy and Safety of Bezafibrate Alone or in Combination with Ursodeoxycholic Acid in Primary Biliary Cholangitis: Systematic Review and Meta-Analysis.Dig Dis Sci. 2023 Apr;68(4):1559-1573. doi: 10.1007/s10620-022-07704-4. Epub 2022 Sep 30. Dig Dis Sci. 2023. PMID: 36180756
-
Management of Primary Biliary Cholangitis: Current Treatment and Future Perspectives.Turk J Gastroenterol. 2023 Feb;34(2):89-100. doi: 10.5152/tjg.2023.22239. Turk J Gastroenterol. 2023. PMID: 36843300 Free PMC article.
-
Primary Biliary Cholangitis and Primary Sclerosing Cholangitis: Current Knowledge of Pathogenesis and Therapeutics.Biomedicines. 2022 May 31;10(6):1288. doi: 10.3390/biomedicines10061288. Biomedicines. 2022. PMID: 35740310 Free PMC article. Review.
-
Pharmacological interventions for primary biliary cholangitis: an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 28;3(3):CD011648. doi: 10.1002/14651858.CD011648.pub2. Cochrane Database Syst Rev. 2017. PMID: 28350426 Free PMC article.
-
Efficacy and safety of PPAR agonists in primary biliary cholangitis: a systematic review and meta-analysis of Randomized Controlled Trials.BMC Gastroenterol. 2025 Apr 8;25(1):230. doi: 10.1186/s12876-025-03821-2. BMC Gastroenterol. 2025. PMID: 40200180 Free PMC article.
References
-
- Prince MI, James OF. The epidemiology of primary biliary cirrhosis. Clin Liver Dis. 2003;7:795–819. - PubMed
-
- Gong Y, Klingenberg SL, Gluud C. Systematic review and meta-analysis: D-Penicillamine vs placebo/no intervention in patients with primary biliary cirrhosis – Cochrane Hepato-Biliary Group. Aliment Pharmacol Ther. 2006;24(11–12):1535–1544. - PubMed
-
- Giljaca V, Poropat G, Stimac D, Gluud C. Methotrexate for primary biliary cirrhosis. Cochrane Database Syst Rev. 2010;5:CD004385. - PubMed
-
- Gong Y, Gluud C. Colchicine for primary biliary cirrhosis: a Cochrane Hepato-Biliary Group systematic review of randomized clinical trials. Am J Gastroenterol. 2005;100(8):1876–1885. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

