A proposal for an individualized pharmacogenetic-guided isoniazid dosage regimen for patients with tuberculosis
- PMID: 26491254
- PMCID: PMC4598210
- DOI: 10.2147/DDDT.S87131
A proposal for an individualized pharmacogenetic-guided isoniazid dosage regimen for patients with tuberculosis
Abstract
Background/aim: Isoniazid (INH) is an essential component of first-line anti-tuberculosis (TB) treatment. However, treatment with INH is complicated by polymorphisms in the expression of the enzyme system primarily responsible for its elimination, N-acetyltransferase 2 (NAT2), and its associated hepatotoxicity. The objective of this study was to develop an individualized INH dosing regimen using a pharmacogenetic-driven model and to apply this regimen in a pilot study.
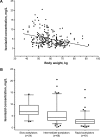
Methods: A total of 206 patients with TB who received anti-TB treatment were included in this prospective study. The 2-hour post-dose concentrations of INH were obtained, and their NAT2 genotype was determined using polymerase chain reaction and sequencing. A multivariate regression analysis that included the variables of age, sex, body weight, and NAT2 genotype was performed to determine the best model for estimating the INH dose that achieves a concentration of 3.0-6.0 mg/L. This dosing algorithm was then used for newly enrolled 53 patients.
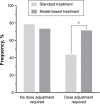
Results: Serum concentrations of INH were significantly lower in the rapid-acetylators than in the slow-acetylators (2.55 mg/L vs 6.78 mg/L, median, P<0.001). A multivariate stepwise linear regression analysis revealed that NAT2 and body weight independently affected INH concentrations: INH concentration (mg/L) = 13.821-0.1× (body weight, kg) -2.273× (number of high activity alleles of NAT2; 0, 1, 2). In 53 newly enrolled patients, the frequency at which they were within the therapeutic range of 3.0-6.0 mg/L was higher in the model-based treatment group compared to the standard treatment group (80.8% vs 59.3%).
Conclusion: The use of individualized pharmacogenetic-guided INH dosage regimens that incorporate NAT2 genotype and body weight may help to ensure achievement of therapeutic concentrations of INH in the TB patients.
Keywords: INH regimen; NAT2 genotype; pharmacogenomics; tuberculosis.
Figures
Similar articles
-
NAT2 genotype guided regimen reduces isoniazid-induced liver injury and early treatment failure in the 6-month four-drug standard treatment of tuberculosis: a randomized controlled trial for pharmacogenetics-based therapy.Eur J Clin Pharmacol. 2013 May;69(5):1091-101. doi: 10.1007/s00228-012-1429-9. Epub 2012 Nov 14. Eur J Clin Pharmacol. 2013. PMID: 23150149 Free PMC article. Clinical Trial.
-
Population Pharmacokinetic Analysis of Isoniazid among Pulmonary Tuberculosis Patients from China.Antimicrob Agents Chemother. 2020 Feb 21;64(3):e01736-19. doi: 10.1128/AAC.01736-19. Print 2020 Feb 21. Antimicrob Agents Chemother. 2020. PMID: 31907179 Free PMC article.
-
Anthropometric and Genetic Factors Associated With the Exposure of Rifampicin and Isoniazid in Mexican Patients With Tuberculosis.Ther Drug Monit. 2019 Oct;41(5):648-656. doi: 10.1097/FTD.0000000000000631. Ther Drug Monit. 2019. PMID: 30939588
-
A Systematic Review and Meta-analysis of Isoniazid Pharmacokinetics in Healthy Volunteers and Patients with Tuberculosis.Clin Ther. 2020 Nov;42(11):e220-e241. doi: 10.1016/j.clinthera.2020.09.009. Epub 2020 Oct 5. Clin Ther. 2020. PMID: 33032843
-
Pharmacogenomic insights into tuberculosis treatment shows the NAT2 genetic variants linked to hepatotoxicity risk: a systematic review and meta-analysis.BMC Genom Data. 2024 Dec 5;25(1):103. doi: 10.1186/s12863-024-01286-y. BMC Genom Data. 2024. PMID: 39639188 Free PMC article.
Cited by
-
Association and clinical utility of NAT2 in the prediction of isoniazid-induced liver injury in Singaporean patients.PLoS One. 2017 Oct 16;12(10):e0186200. doi: 10.1371/journal.pone.0186200. eCollection 2017. PLoS One. 2017. PMID: 29036176 Free PMC article.
-
Pharmacogenetics as part of recommended precision medicine for tuberculosis treatment in African populations: Could it be a reality?Clin Transl Sci. 2023 Jul;16(7):1101-1112. doi: 10.1111/cts.13520. Epub 2023 Jun 8. Clin Transl Sci. 2023. PMID: 37291686 Free PMC article. Review.
-
The role of isoniazid dosage and NAT2 gene polymorphism in the treatment of tuberculous meningitis.Front Immunol. 2025 Jan 20;15:1535447. doi: 10.3389/fimmu.2024.1535447. eCollection 2024. Front Immunol. 2025. PMID: 39902038 Free PMC article.
-
Arylamine N-acetyltransferase acetylation polymorphisms: paradigm for pharmacogenomic-guided therapy- a focused review.Expert Opin Drug Metab Toxicol. 2021 Jan;17(1):9-21. doi: 10.1080/17425255.2021.1840551. Epub 2020 Nov 3. Expert Opin Drug Metab Toxicol. 2021. PMID: 33094670 Free PMC article. Review.
-
Clinical Pharmacogenetic Testing and Application: Laboratory Medicine Clinical Practice Guidelines.Ann Lab Med. 2017 Mar;37(2):180-193. doi: 10.3343/alm.2017.37.2.180. Ann Lab Med. 2017. PMID: 28029011 Free PMC article.
References
-
- World Health Organization Global Health Observatory Tuberculosis. [Accessed February 21, 2015]. Available from: http://www.who.int/gho/tb/en/index.html.
-
- World Health Organization Guidelines for Treatment of Tuberculosis. [Accessed February 23, 2015]. Available from: http://www.who.int/tb/publications/2010/9789241547833/en/
-
- Joint Committee for the Development of Korean Guidelines for TB and Korea Centers for Disease Control and Prevention . Korean Guidelines for Tuberculosis. 2014.
-
- Du H, Chen X, Fang Y, et al. Slow N-acetyltransferase 2 genotype contributes to anti-tuberculosis drug-induced hepatotoxicity: a meta-analysis. Mol Biol Rep. 2013;40(5):3591–3596. - PubMed
-
- Wang JY, Liu CH, Hu FC, et al. Risk factors of hepatitis during anti-tuberculous treatment and implications of hepatitis virus load. J Infect. 2011;62(6):448–455. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

