An efficient PEGylated liposomal nanocarrier containing cell-penetrating peptide and pH-sensitive hydrazone bond for enhancing tumor-targeted drug delivery
- PMID: 26491292
- PMCID: PMC4598226
- DOI: 10.2147/IJN.S92519
An efficient PEGylated liposomal nanocarrier containing cell-penetrating peptide and pH-sensitive hydrazone bond for enhancing tumor-targeted drug delivery
Abstract
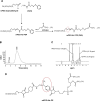
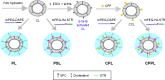
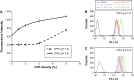
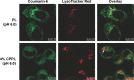
Cell-penetrating peptides (CPPs) as small molecular transporters with abilities of cell penetrating, internalization, and endosomal escape have potential prospect in drug delivery systems. However, a bottleneck hampering their application is the poor specificity for cells. By utilizing the function of hydration shell of polyethylene glycol (PEG) and acid sensitivity of hydrazone bond, we constructed a kind of CPP-modified pH-sensitive PEGylated liposomes (CPPL) to improve the selectivity of these peptides for tumor targeting. In CPPL, CPP was directly attached to liposome surfaces via coupling with stearate (STR) to avoid the hindrance of PEG as a linker on the penetrating efficiency of CPP. A PEG derivative by conjugating PEG with STR via acid-degradable hydrazone bond (PEG2000-Hz-STR, PHS) was synthesized. High-performance liquid chromatography and flow cytometry demonstrated that PHS was stable at normal neutral conditions and PEG could be completely cleaved from liposome surface to expose CPP under acidic environments in tumor. An optimal CPP density on liposomes was screened to guaranty a maximum targeting efficiency on tumor cells as well as not being captured by normal cells that consequently lead to a long circulation in blood. In vitro and in vivo studies indicated, in 4 mol% CPP of lipid modified system, that CPP exerted higher efficiency on internalizing the liposomes into targeted subcellular compartments while remaining inactive and free from opsonins at a maximum extent in systemic circulation. The 4% CPPL as a drug delivery system will have great potential in the clinical application of anticancer drugs in future.
Keywords: long circulation; lysosome escape; nanocarrier; pH-sensitive liposomes; pharmacokinetics.
Figures



References
-
- Chiu SJ, Liu S, Perrotti D, Marcucci G, Lee RJ. Efficient delivery of a Bcl-2-specific antisense oligodeoxyribonucleotide (G3139) via transferrin receptor-targeted liposomes. J Control Release. 2006;112(2):199–207. - PubMed
-
- Kalyanasundaram K, Thomas JK. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc. 1977;99(7):2039–2044.
-
- Aguiar J, Carpena P, Molina-Bolívar JA, Carnero Ruiz C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J Colloid Interface Sci. 2003;258(1):116–122.
-
- Cullis PR, Chonn A. Recent advances in liposome technologies and their applications for systemic gene delivery. Adv Drug Deliv Rev. 1998;30(1–3):73–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

