Sex Hormones and Their Receptors Regulate Liver Energy Homeostasis
- PMID: 26491440
- PMCID: PMC4600502
- DOI: 10.1155/2015/294278
Sex Hormones and Their Receptors Regulate Liver Energy Homeostasis
Abstract
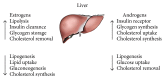
The liver is one of the most essential organs involved in the regulation of energy homeostasis. Hepatic steatosis, a major manifestation of metabolic syndrome, is associated with imbalance between lipid formation and breakdown, glucose production and catabolism, and cholesterol synthesis and secretion. Epidemiological studies show sex difference in the prevalence in fatty liver disease and suggest that sex hormones may play vital roles in regulating hepatic steatosis. In this review, we summarize current literature and discuss the role of estrogens and androgens and the mechanisms through which estrogen receptors and androgen receptors regulate lipid and glucose metabolism in the liver. In females, estradiol regulates liver metabolism via estrogen receptors by decreasing lipogenesis, gluconeogenesis, and fatty acid uptake, while enhancing lipolysis, cholesterol secretion, and glucose catabolism. In males, testosterone works via androgen receptors to increase insulin receptor expression and glycogen synthesis, decrease glucose uptake and lipogenesis, and promote cholesterol storage in the liver. These recent integrated concepts suggest that sex hormone receptors could be potential promising targets for the prevention of hepatic steatosis.
Figures

References
-
- Martensson U. E. A., Salehi S. A., Windahl S., et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150(2):687–698. doi: 10.1210/en.2008-0623. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

