Alleviation of Carbon-Tetrachloride-Induced Liver Injury and Fibrosis by Betaine Supplementation in Chickens
- PMID: 26491462
- PMCID: PMC4600548
- DOI: 10.1155/2015/725379
Alleviation of Carbon-Tetrachloride-Induced Liver Injury and Fibrosis by Betaine Supplementation in Chickens
Abstract
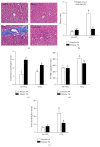
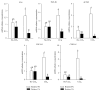
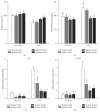
Betaine is a food component with well-reported hepatoprotection effects. However, the effects and mechanisms of betaine on liver fibrosis development are still insufficient. Because metabolic functions of chicken and human liver is similar, we established a chicken model with carbon Tetrachloride- (CCl4-) induced fibrosis for studying antifibrotic effect of betaine in vivo and in vitro. Two-week-old male chicks were supplemented with betaine (1%, w/v) in drinking water for 2 weeks prior to the initiation of CCl4 treatment (i.p.) until sacrifice. Primary chicken hepatocytes were treated with CCl4 and betaine to mimic the in vivo supplementation. The supplementation of betaine significantly alleviated liver fibrosis development along with the inhibition of lipid peroxidation, hepatic inflammation cytokine, and transforming growth factor-β1 expression levels. These inhibitive effects were also accompanied with the attenuation of hepatic stellate cell activation. Furthermore, our in vitro studies confirmed that betaine provides antioxidant capacity for attenuating the hepatocyte necrosis by CCl4. Altogether, our results highlight the antioxidant ability of betaine, which alleviates CCl4-induced fibrogenesis process along with the suppression of hepatic stellate cells activation. Since betaine is a natural compound without toxicity, we suggest betaine can be used as a potent nutritional or therapeutic factor for reducing liver fibrosis.
Figures



Similar articles
-
Betaine treatment decreased oxidative stress, inflammation, and stellate cell activation in rats with alcoholic liver fibrosis.Environ Toxicol Pharmacol. 2016 Jul;45:170-8. doi: 10.1016/j.etap.2016.05.033. Epub 2016 Jun 1. Environ Toxicol Pharmacol. 2016. PMID: 27314760
-
High-fat diet plus carbon tetrachloride-induced liver fibrosis is alleviated by betaine treatment in rats.Int Immunopharmacol. 2016 Oct;39:199-207. doi: 10.1016/j.intimp.2016.07.028. Epub 2016 Aug 3. Int Immunopharmacol. 2016. PMID: 27494683
-
Alleviation of dimethylnitrosamine-induced liver injury and fibrosis by betaine supplementation in rats.Chem Biol Interact. 2009 Feb 12;177(3):204-11. doi: 10.1016/j.cbi.2008.09.021. Epub 2008 Sep 26. Chem Biol Interact. 2009. PMID: 18930038
-
Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model.Crit Rev Toxicol. 2003;33(2):105-36. doi: 10.1080/713611034. Crit Rev Toxicol. 2003. PMID: 12708612 Review.
-
Recent Advances in Betaine: Extraction, Identification, and Bioactive Functions in Human Health.Food Sci Nutr. 2025 Apr 17;13(4):e70173. doi: 10.1002/fsn3.70173. eCollection 2025 Apr. Food Sci Nutr. 2025. PMID: 40255548 Free PMC article. Review.
Cited by
-
Growth Performance and Characterization of Meat Quality of Broiler Chickens Supplemented with Betaine and Antioxidants under Cyclic Heat Stress.Antioxidants (Basel). 2019 Aug 22;8(9):336. doi: 10.3390/antiox8090336. Antioxidants (Basel). 2019. PMID: 31443527 Free PMC article.
-
Betaine and Antioxidants Improve Growth Performance, Breast Muscle Development and Ameliorate Thermoregulatory Responses to Cyclic Heat Exposure in Broiler Chickens.Animals (Basel). 2018 Sep 25;8(10):162. doi: 10.3390/ani8100162. Animals (Basel). 2018. PMID: 30257522 Free PMC article.
-
Highly Water-Preserving Zwitterionic Betaine-Incorporated Collagen Sponges With Anti-oxidation and Anti-inflammation for Wound Regeneration.Front Cell Dev Biol. 2020 Jul 15;8:491. doi: 10.3389/fcell.2020.00491. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32766236 Free PMC article.
-
The Role of Macrophage Inhibitory Factor in TAA-Induced Liver Fibrosis in Mice: Modulatory Effects of Betaine.Biomedicines. 2024 Jun 17;12(6):1337. doi: 10.3390/biomedicines12061337. Biomedicines. 2024. PMID: 38927544 Free PMC article.
-
Dietary Betaine Improves Intestinal Barrier Function and Ameliorates the Impact of Heat Stress in Multiple Vital Organs as Measured by Evans Blue Dye in Broiler Chickens.Animals (Basel). 2019 Dec 23;10(1):38. doi: 10.3390/ani10010038. Animals (Basel). 2019. PMID: 31878074 Free PMC article.
References
-
- Wang C., Zhang T., Cui X., Li S., Zhao X., Zhong X. Hepatoprotective effects of a chinese herbal formula, longyin decoction, on carbon-tetrachloride-induced liver injury in chickens. Evidence-based Complementary and Alternative Medicine. 2013;2013:9. doi: 10.1155/2013/392743.392743 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

