Chemotherapy-Induced Cardiotoxicity: Overview of the Roles of Oxidative Stress
- PMID: 26491536
- PMCID: PMC4602327
- DOI: 10.1155/2015/795602
Chemotherapy-Induced Cardiotoxicity: Overview of the Roles of Oxidative Stress
Abstract
Chemotherapy-induced cardiotoxicity is a serious complication that poses a serious threat to life and limits the clinical use of various chemotherapeutic agents, particularly the anthracyclines. Understanding molecular mechanisms of chemotherapy-induced cardiotoxicity is a key to effective preventive strategies and improved chemotherapy regimen. Although no reliable and effective preventive treatment has become available, numerous evidence demonstrates that chemotherapy-induced cardiotoxicity involves the generation of reactive oxygen species (ROS). This review provides an overview of the roles of oxidative stress in chemotherapy-induced cardiotoxicity using doxorubicin, which is one of the most effective chemotherapeutic agents against a wide range of cancers, as an example. Current understanding in the molecular mechanisms of ROS-mediated cardiotoxicity will be explored and discussed, with emphasis on cardiomyocyte apoptosis leading to cardiomyopathy. The review will conclude with perspectives on model development needed to facilitate further progress and understanding on chemotherapy-induced cardiotoxicity.
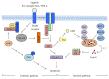
Figures

References
-
- Kufe D. W., Holland J. F., Frei E. American Cancer Society: Cancer Medicine. Hamilton, Canada: BC Decker; 2003.
-
- Hershman D. L., McBride R. B., Eisenberger A., Wei Y. T., Grann V. R., Jacobson J. S. Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin's lymphoma. Journal of Clinical Oncology. 2008;26(19):3159–3165. doi: 10.1200/jco.2007.14.1242. - DOI - PubMed
-
- Gianni L., Munzone E., Capri G., et al. Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: high antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. Journal of Clinical Oncology. 1995;13(11):2688–2699. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

