Gated Silica Mesoporous Materials in Sensing Applications
- PMID: 26491626
- PMCID: PMC4603401
- DOI: 10.1002/open.201500053
Gated Silica Mesoporous Materials in Sensing Applications
Abstract
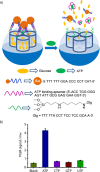
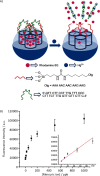
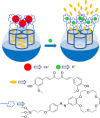
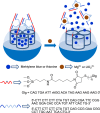
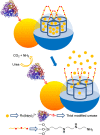
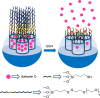
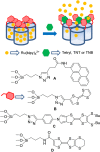
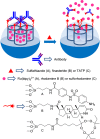
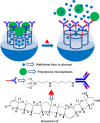
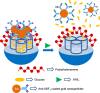
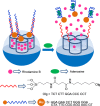
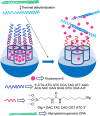
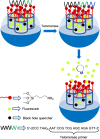
Silica mesoporous supports (SMSs) have a large specific surface area and volume and are particularly exciting vehicles for delivery applications. Such container-like structures can be loaded with numerous different chemical substances, such as drugs and reporters. Gated systems also contain addressable functions at openings of voids, and cargo delivery can be controlled on-command using chemical, biochemical or physical stimuli. Many of these gated SMSs have been applied for drug delivery. However, fewer examples of their use in sensing protocols have been reported. The approach of applying SMSs in sensing uses another concept-that of loading pores with a reporter and designing a capping mechanism that is selectively opened in the presence of a target analyte, which results in the delivery of the reporter. According to this concept, we provide herein a complete compilation of published examples of probes based on the use of capped SMSs for sensing. Examples for the detection of anions, cations, small molecules and biomolecules are provided. The diverse range of gated silica mesoporous materials presented here highlights their usefulness in recognition protocols.
Keywords: anions; biomolecules; cations; gated materials; mesoporous silica; sensors.
Figures


















Similar articles
-
Gated silica mesoporous supports for controlled release and signaling applications.Acc Chem Res. 2013 Feb 19;46(2):339-49. doi: 10.1021/ar3001469. Epub 2012 Dec 6. Acc Chem Res. 2013. PMID: 23214509
-
New Advances in In Vivo Applications of Gated Mesoporous Silica as Drug Delivery Nanocarriers.Small. 2020 Jan;16(3):e1902242. doi: 10.1002/smll.201902242. Epub 2019 Dec 17. Small. 2020. PMID: 31846230 Review.
-
Fluorogenic Sensing of Carcinogenic Bisphenol A using Aptamer-Capped Mesoporous Silica Nanoparticles.Chemistry. 2017 Jun 27;23(36):8581-8584. doi: 10.1002/chem.201701024. Epub 2017 Jun 5. Chemistry. 2017. PMID: 28498545
-
Optimization of analytical assay performance of antibody-gated indicator-releasing mesoporous silica particles.J Mater Chem B. 2020 Jun 10;8(22):4950-4961. doi: 10.1039/d0tb00371a. J Mater Chem B. 2020. PMID: 32469027
-
Multi-functional silica-based mesoporous materials for simultaneous delivery of biologically active ions and therapeutic biomolecules.Acta Biomater. 2021 Jul 15;129:1-17. doi: 10.1016/j.actbio.2021.05.007. Epub 2021 May 16. Acta Biomater. 2021. PMID: 34010692 Review.
Cited by
-
Not always what closes best opens better: mesoporous nanoparticles capped with organic gates.Sci Technol Adv Mater. 2019 Jun 26;20(1):699-709. doi: 10.1080/14686996.2019.1627173. eCollection 2019. Sci Technol Adv Mater. 2019. PMID: 31275461 Free PMC article.
-
Bacteriophage-Gated Optical Sensor for Bacteria Detection.Anal Chem. 2025 Jul 1;97(25):13103-13109. doi: 10.1021/acs.analchem.5c00780. Epub 2025 May 23. Anal Chem. 2025. PMID: 40408168 Free PMC article.
-
Biosensors for Cancer Biomarkers Based on Mesoporous Silica Nanoparticles.Biosensors (Basel). 2024 Jun 30;14(7):326. doi: 10.3390/bios14070326. Biosensors (Basel). 2024. PMID: 39056602 Free PMC article. Review.
-
Functional Magnetic Mesoporous Silica Microparticles Capped with an Azo-Derivative: A Promising Colon Drug Delivery Device.Molecules. 2018 Feb 10;23(2):375. doi: 10.3390/molecules23020375. Molecules. 2018. PMID: 29439396 Free PMC article.
-
Gene-Directed Enzyme Prodrug Therapy by Dendrimer-Like Mesoporous Silica Nanoparticles against Tumor Cells.Nanomaterials (Basel). 2021 May 14;11(5):1298. doi: 10.3390/nano11051298. Nanomaterials (Basel). 2021. PMID: 34069171 Free PMC article.
References
-
- Katz E, Willner I. Angew. Chem. Int. Ed. 2004;43:6042–6108. - PubMed
- Angew. Chem. 2004;116
-
- Wang F, Liu X, Willner I. Angew. Chem. Int. Ed. 2015;54:1098–1129. - PubMed
- Angew. Chem. 2015;127
-
- Descalzo AB, Martínez-Máñez R, Sancnón F, Hoffmann K, Rurack K. Angew. Chem. Int. Ed. 2006;45:5924–5948. - PubMed
- Angew. Chem. 2006;118
-
- Lu CH, Willner B, Willner I. ACS Nano. 2013;7:8320–8332. - PubMed
-
- Saha S, Leung KCF, Nguyen TD, Stoddart JF, Zink JI. Adv. Funct. Mater. 2007;17:685–693.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

