Synthesis, Characterization, and Biological Evaluation of a Dual-Action Ligand Targeting αvβ3 Integrin and VEGF Receptors
- PMID: 26491644
- PMCID: PMC4608532
- DOI: 10.1002/open.201500062
Synthesis, Characterization, and Biological Evaluation of a Dual-Action Ligand Targeting αvβ3 Integrin and VEGF Receptors
Abstract
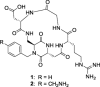
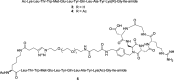
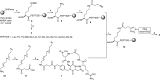
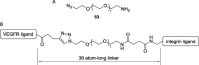
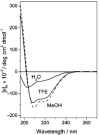
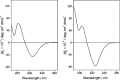
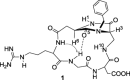
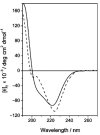
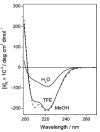
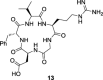
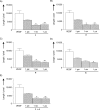
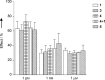
A dual-action ligand targeting both integrin αVβ3 and vascular endothelial growth factor receptors (VEGFRs), was synthesized via conjugation of a cyclic peptidomimetic αVβ3 Arg-Gly-Asp (RGD) ligand with a decapentapeptide. The latter was obtained from a known VEGFR antagonist by acetylation at the Lys13 side chain. Functionalization of the precursor ligands was carried out in solution and in the solid phase, affording two fragments: an alkyne VEGFR ligand and the azide integrin αVβ3 ligand, which were conjugated by click chemistry. Circular dichroism studies confirmed that both the RGD and VEGFR ligand portions of the dual-action compound substantially adopt the biologically active conformation. In vitro binding assays on isolated integrin αVβ3 and VEGFR-1 showed that the dual-action conjugate retains a good level of affinity for both its target receptors, although with one order of magnitude (10/20 times) decrease in potency. The dual-action ligand strongly inhibited the VEGF-induced morphogenesis in Human Umbilical Vein Endothelial Cells (HUVECs). Remarkably, its efficiency in preventing the formation of new blood vessels was similar to that of the original individual ligands, despite the worse affinity towards integrin αVβ3 and VEGFR-1.
Keywords: Angiogenesis; VEGFR; dual-action ligands; integrins; ligand conjugation.
Figures

Similar articles
-
FAS1 domain protein inhibits VEGF165-induced angiogenesis by targeting the interaction between VEGFR-2 and αvβ3 integrin.Mol Cancer Res. 2012 Aug;10(8):1010-20. doi: 10.1158/1541-7786.MCR-11-0600. Epub 2012 Jun 18. Mol Cancer Res. 2012. PMID: 22710795
-
Imaging VEGF Receptors and αvβ3 Integrins in a Mouse Hindlimb Ischemia Model of Peripheral Arterial Disease.Mol Imaging Biol. 2018 Dec;20(6):963-972. doi: 10.1007/s11307-018-1191-1. Mol Imaging Biol. 2018. PMID: 29687324
-
Glycation of vitronectin inhibits VEGF-induced angiogenesis by uncoupling VEGF receptor-2-αvβ3 integrin cross-talk.Cell Death Dis. 2015 Jun 25;6(6):e1796. doi: 10.1038/cddis.2015.174. Cell Death Dis. 2015. PMID: 26111058 Free PMC article.
-
Small molecule hormone or hormone-like ligands of integrin αVβ3: implications for cancer cell behavior.Horm Cancer. 2013 Dec;4(6):335-42. doi: 10.1007/s12672-013-0156-8. Epub 2013 Aug 14. Horm Cancer. 2013. PMID: 23943159 Free PMC article. Review.
-
Biological Relevance of RGD-Integrin Subtype-Specific Ligands in Cancer.Chembiochem. 2021 Apr 6;22(7):1151-1160. doi: 10.1002/cbic.202000626. Epub 2020 Nov 27. Chembiochem. 2021. PMID: 33140906 Review.
Cited by
-
Rational Design of Antiangiogenic Helical Oligopeptides Targeting the Vascular Endothelial Growth Factor Receptors.Front Chem. 2019 Mar 29;7:170. doi: 10.3389/fchem.2019.00170. eCollection 2019. Front Chem. 2019. PMID: 30984741 Free PMC article.
-
Novel Small Multilamellar Liposomes Containing Large Quantities of Peptide Nucleic Acid Selectively Kill Breast Cancer Cells.Cancers (Basel). 2022 Sep 30;14(19):4806. doi: 10.3390/cancers14194806. Cancers (Basel). 2022. PMID: 36230729 Free PMC article.
-
Tumor Targeting with an isoDGR-Drug Conjugate.Chemistry. 2017 Jun 12;23(33):7910-7914. doi: 10.1002/chem.201701844. Epub 2017 May 26. Chemistry. 2017. PMID: 28449309 Free PMC article.
-
Multivalency Increases the Binding Strength of RGD Peptidomimetic-Paclitaxel Conjugates to Integrin αV β3.Chemistry. 2017 Oct 17;23(58):14410-14415. doi: 10.1002/chem.201703093. Epub 2017 Sep 6. Chemistry. 2017. PMID: 28816404 Free PMC article.
-
Review of Integrin-Targeting Biomaterials in Tissue Engineering.Adv Healthc Mater. 2020 Dec;9(23):e2000795. doi: 10.1002/adhm.202000795. Epub 2020 Sep 16. Adv Healthc Mater. 2020. PMID: 32940020 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

