Influence of Ionizing Radiation on Two Generations of Cochlear Implants
- PMID: 26491679
- PMCID: PMC4600872
- DOI: 10.1155/2015/609607
Influence of Ionizing Radiation on Two Generations of Cochlear Implants
Abstract
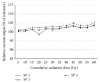
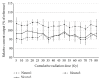
The purpose of the present study was to test the behavior of two different generations of cochlear implant systems subjected to a clinical radiotherapy scheme and to determine the maximal acceptable cumulative radiation levels at which the devices show out-of-specification behaviors. Using stereotactic irradiation (Cyberknife, 6 MV photon beam), three Digisonic SP and three Neuro devices were submitted to 5 Gy doses that cumulated to 60 Gy (12 sessions) and 80 Gy (16 sessions), respectively. A follow-up series of irradiation was then applied, in which Digisonic SP devices received two additional fractions of 50 Gy each, cumulating to 160 Gy, and Neuro devices three additional fractions of 20, 40, and 150 Gy, cumulating to 290 Gy. Output current values were monitored during the treatment. At clinical doses, with 60 or 80 Gy cumulative radiation exposure, no single measurement showed more than 10% divergence from the reference measure. The cochlear implants tested in this study showed high resistance to clinically relevant cumulative radiation doses and showed no out-of-bounds behavior up to cumulative doses of 140 or 160 Gy. These observations suggest that cochlear implant users can undergo radiotherapy up to cumulative doses well above those currently used in clinical situations without risk of failure.
Figures


Similar articles
-
The influence of ionizing radiation on the CLARION 1.2 cochlear implant during radiation therapy.Am J Otol. 1999 Jan;20(1):50-2. Am J Otol. 1999. PMID: 9918172
-
Influence of ionizing radiation on nucleus 24 cochlear implants.Otol Neurotol. 2005 Jul;26(4):661-7. doi: 10.1097/01.mao.0000178134.96977.f5. Otol Neurotol. 2005. PMID: 16015164
-
Cochlear implants: response to therapeutic irradiation.Int J Radiat Oncol Biol Phys. 1999 Apr 1;44(1):227-31. doi: 10.1016/s0360-3016(98)00532-x. Int J Radiat Oncol Biol Phys. 1999. PMID: 10219818
-
The effect of large single radiation doses on cochlear implant function: implications for radiosurgery.Eur Arch Otorhinolaryngol. 2004 May;261(5):251-5. doi: 10.1007/s00405-003-0670-3. Epub 2003 Sep 18. Eur Arch Otorhinolaryngol. 2004. PMID: 14504862
-
Reirradiation tolerance of the human brain.Int J Radiat Oncol Biol Phys. 2008 Apr 1;70(5):1350-60. doi: 10.1016/j.ijrobp.2007.08.015. Epub 2007 Nov 26. Int J Radiat Oncol Biol Phys. 2008. PMID: 18037587 Review.
Cited by
-
Challenges of Hearing Rehabilitation after Radiation and Chemotherapy.J Neurol Surg B Skull Base. 2019 Apr;80(2):214-224. doi: 10.1055/s-0039-1677865. Epub 2019 Feb 4. J Neurol Surg B Skull Base. 2019. PMID: 30931231 Free PMC article.
References
-
- Hashii H., Hashimoto T., Okawa A., et al. Comparison of the effects of high-energy photon beam irradiation (10 and 18 MV) on 2 types of implantable cardioverter-defibrillators. International Journal of Radiation Oncology Biology Physics. 2013;85(3):840–845. doi: 10.1016/j.ijrobp.2012.05.043. - DOI - PubMed
-
- Baumann R., Lesinski-Schiedat A., Goldring J. E., et al. The influence of ionizing radiation on the CLARION 1.2 cochlear implant during radiation therapy. American Journal of Otology. 1999;20(1):50–52. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

