Roles of Akt and SGK1 in the Regulation of Renal Tubular Transport
- PMID: 26491696
- PMCID: PMC4600925
- DOI: 10.1155/2015/971697
Roles of Akt and SGK1 in the Regulation of Renal Tubular Transport
Abstract
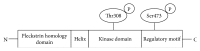
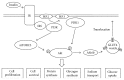
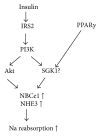
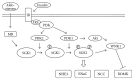
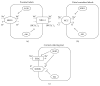
A serine/threonine kinase Akt is a key mediator in various signaling pathways including regulation of renal tubular transport. In proximal tubules, Akt mediates insulin signaling via insulin receptor substrate 2 (IRS2) and stimulates sodium-bicarbonate cotransporter (NBCe1), resulting in increased sodium reabsorption. In insulin resistance, the IRS2 in kidney cortex is exceptionally preserved and may mediate the stimulatory effect of insulin on NBCe1 to cause hypertension in diabetes via sodium retention. Likewise, in distal convoluted tubules and cortical collecting ducts, insulin-induced Akt phosphorylation mediates several hormonal signals to enhance sodium-chloride cotransporter (NCC) and epithelial sodium channel (ENaC) activities, resulting in increased sodium reabsorption. Serum- and glucocorticoid-inducible kinase 1 (SGK1) mediates aldosterone signaling. Insulin can stimulate SGK1 to exert various effects on renal transporters. In renal cortical collecting ducts, SGK1 regulates the expression level of ENaC through inhibition of its degradation. In addition, SGK1 and Akt cooperatively regulate potassium secretion by renal outer medullary potassium channel (ROMK). Moreover, sodium-proton exchanger 3 (NHE3) in proximal tubules is possibly activated by SGK1. This review focuses on recent advances in understanding of the roles of Akt and SGK1 in the regulation of renal tubular transport.
Figures
References
-
- Staal S. P. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(14):5034–5037. doi: 10.1073/pnas.84.14.5034. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

