Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients
- PMID: 26491796
- PMCID: PMC4688194
- DOI: 10.1097/TA.0000000000000885
Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients
Abstract
Background: Trauma-induced coagulopathy (TIC) is associated with a fourfold increased risk of mortality. Hyperfibrinolysis is a component of TIC, but its mechanism is poorly understood. Plasminogen activation inhibitor (PAI-1) degradation by activated protein C has been proposed as a mechanism for deregulation of the plasmin system in hemorrhagic shock, but in other settings of ischemia, tissue plasminogen activator (tPA) has been shown to be elevated. We hypothesized that the hyperfibrinolysis in TIC is not the result of PAI-1 degradation but is driven by an increase in tPA, with resultant loss of PAI-1 activity through complexation with tPA.
Methods: Eighty-six consecutive trauma activation patients had blood collected at the earliest time after injury and were screened for hyperfibrinolysis using thrombelastography (TEG). Twenty-five hyperfibrinolytic patients were compared with 14 healthy controls using enzyme-linked immunosorbent assays for active tPA, active PAI-1, and PAI-1/tPA complex. Blood was also subjected to TEG with exogenous tPA challenge as a functional assay for PAI-1 reserve.
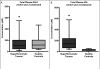
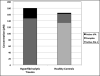
Results: Total levels of PAI-1 (the sum of the active PAI-1 species and its covalent complex with tPA) are not significantly different between hyperfibrinolytic trauma patients and healthy controls: median, 104 pM (interquartile range [IQR], 48-201 pM) versus 115 pM (IQR, 54-202 pM). The ratio of active to complexed PAI-1, however, was two orders of magnitude lower in hyperfibrinolytic patients than in controls. Conversely, total tPA levels (active + complex) were significantly higher in hyperfibrinolytic patients than in controls: 139 pM (IQR, 68-237 pM) versus 32 pM (IQR, 16-37 pM). Hyperfibrinolytic trauma patients displayed increased sensitivity to exogenous challenge with tPA (median LY30 of 66.8% compared with 9.6% for controls).
Conclusion: Depletion of PAI-1 in TIC is driven by an increase in tPA, not PAI-1 degradation. The tPA-challenged TEG, based on this principle, is a functional test for PAI-1 reserves. Exploration of the mechanism of up-regulation of tPA is critical to an understanding of hyperfibrinolysis in trauma.
Level of evidence: Prognostic and epidemiologic study, level II.
Figures




References
-
- Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003;17(3):223–31. - PubMed
-
- Blackbourne LH, Baer DG, Cestero RF, Inaba K, Rasmussen TE. Exsanguination shock: the next frontier in prevention of battlefield mortality. J Trauma. 2011;71(1 Suppl):S1–3. - PubMed
-
- Ganter MT, Pittet JF. New insights into acute coagulopathy in trauma patients. Best Pract Res Clin Anaesthesiol. 2010;24(1):15–25. - PubMed
-
- Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112–9. - PubMed
-
- Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65(2):261–70. discussion 70-1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

