FGFR3/fibroblast growth factor receptor 3 inhibits autophagy through decreasing the ATG12-ATG5 conjugate, leading to the delay of cartilage development in achondroplasia
- PMID: 26491898
- PMCID: PMC4824585
- DOI: 10.1080/15548627.2015.1091551
FGFR3/fibroblast growth factor receptor 3 inhibits autophagy through decreasing the ATG12-ATG5 conjugate, leading to the delay of cartilage development in achondroplasia
Abstract
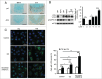
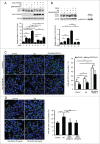
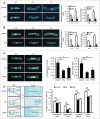
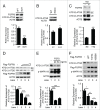
FGFR3 (fibroblast growth factor receptor 3) is a negative regulator of endochondral ossification. Gain-of-function mutations in FGFR3 are responsible for achondroplasia, the most common genetic form of dwarfism in humans. Autophagy, an evolutionarily conserved catabolic process, maintains chondrocyte viability in the growth plate under stress conditions, such as hypoxia and nutritional deficiencies. However, the role of autophagy and its underlying molecular mechanisms in achondroplasia remain elusive. In this study, we found activated FGFR3 signaling inhibited autophagic activity in chondrocytes, both in vivo and in vitro. By employing an embryonic bone culture system, we demonstrated that treatment with autophagy inhibitor 3-MA or chloroquine led to cartilage growth retardation, which mimics the effect of activated-FGFR3 signaling on chondrogenesis. Furthermore, we found that FGFR3 interacted with ATG12-ATG5 conjugate by binding to ATG5. More intriguingly, FGFR3 signaling was found to decrease the protein level of ATG12-ATG5 conjugate. Consistently, using in vitro chondrogenic differentiation assay system, we showed that the ATG12-ATG5 conjugate was essential for the viability and differentiation of chondrocytes. Transient transfection of ATG5 partially rescued FGFR3-mediated inhibition on chondrocyte viability and differentiation. Our findings reveal that FGFR3 inhibits the autophagic activity by decreasing the ATG12-ATG5 conjugate level, which may play an essential role in the pathogenesis of achondroplasia.
Keywords: ATG12–ATG5 conjugate; FGFR3; achondroplasia; autophagy; chondrocytes.
Figures

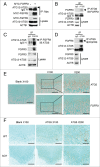
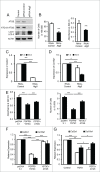
Similar articles
-
FGFR3 induces degradation of BMP type I receptor to regulate skeletal development.Biochim Biophys Acta. 2014 Jul;1843(7):1237-47. doi: 10.1016/j.bbamcr.2014.03.011. Epub 2014 Mar 20. Biochim Biophys Acta. 2014. PMID: 24657641 Free PMC article.
-
Repression of hedgehog signaling and BMP4 expression in growth plate cartilage by fibroblast growth factor receptor 3.Development. 1998 Dec;125(24):4977-88. doi: 10.1242/dev.125.24.4977. Development. 1998. PMID: 9811582
-
Constitutive activation of MEK1 in chondrocytes causes Stat1-independent achondroplasia-like dwarfism and rescues the Fgfr3-deficient mouse phenotype.Genes Dev. 2004 Feb 1;18(3):290-305. doi: 10.1101/gad.1179104. Genes Dev. 2004. PMID: 14871928 Free PMC article.
-
Achondroplasia: Development, pathogenesis, and therapy.Dev Dyn. 2017 Apr;246(4):291-309. doi: 10.1002/dvdy.24479. Epub 2017 Mar 2. Dev Dyn. 2017. PMID: 27987249 Free PMC article. Review.
-
C-Type Natriuretic Peptide Analog as Therapy for Achondroplasia.Endocr Dev. 2016;30:98-105. doi: 10.1159/000439334. Epub 2015 Dec 10. Endocr Dev. 2016. PMID: 26684019 Review.
Cited by
-
Fgfr3 mutation disrupts chondrogenesis and bone ossification in zebrafish model mimicking CATSHL syndrome partially via enhanced Wnt/β-catenin signaling.Theranostics. 2020 May 30;10(16):7111-7130. doi: 10.7150/thno.45286. eCollection 2020. Theranostics. 2020. PMID: 32641982 Free PMC article.
-
Oestrogen-activated autophagy has a negative effect on the anti-osteoclastogenic function of oestrogen.Cell Prolif. 2020 Apr;53(4):e12789. doi: 10.1111/cpr.12789. Epub 2020 Mar 11. Cell Prolif. 2020. PMID: 32157750 Free PMC article.
-
Il-1β Promotes Superficial Zone Cells Senescence in Articular Cartilage by Inhibiting Autophagy.Cartilage. 2024 Dec;15(4):428-439. doi: 10.1177/19476035231194771. Epub 2023 Aug 31. Cartilage. 2024. PMID: 37650417 Free PMC article.
-
The Aberrant Expression of the Mesenchymal Variant of FGFR2 in the Epithelial Context Inhibits Autophagy.Cells. 2019 Jun 29;8(7):653. doi: 10.3390/cells8070653. Cells. 2019. PMID: 31261937 Free PMC article.
-
FGF2/FGFR1 regulates autophagy in FGFR1-amplified non-small cell lung cancer cells.J Exp Clin Cancer Res. 2017 May 30;36(1):72. doi: 10.1186/s13046-017-0534-0. J Exp Clin Cancer Res. 2017. PMID: 28558758 Free PMC article.
References
-
- Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harb Perspect Biol 2013; 5:a008334; PMID:23284041; http://dx.doi.org/10.1101/cshperspect.a008334 - DOI - PMC - PubMed
-
- Erlebacher A, Filvaroff EH, Gitelman SE, Derynck R. Toward a molecular understanding of skeletal development. Cell 1995; 80:371-8; PMID:7859279; http://dx.doi.org/10.1016/0092-8674(95)90487-5 - DOI - PubMed
-
- De Luca F, Baron J. Control of bone growth by fibroblast growth factors. Trends Endocrinol Metab 1999; 10:61-5; PMID:10322396; http://dx.doi.org/10.1016/S1043-2760(98)00120-9 - DOI - PubMed
-
- Bonaventure J, Rousseau F, Legeai-Mallet L, Le Merrer M, Munnich A, Maroteaux P. Common mutations in the gene encoding fibroblast growth factor receptor 3 account for achondroplasia, hypochondroplasia and thanatophoric dysplasia. Acta Paediatr Suppl 1996; 417:33-8; PMID:9055906; http://dx.doi.org/10.1111/j.1651-2227.1996.tb14291.x - DOI - PubMed
-
- Rousseau F, Bonaventure J, Legeai-Mallet L, Pelet A, Rozet JM, Maroteaux P, Le Merrer M, Munnich A. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature 1994; 371:252-4; PMID:8078586; http://dx.doi.org/10.1038/371252a0 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
