Clinical, Radiographic, and Pathologic Findings in Patients Undergoing Reoperation Following Radiation Therapy and Temozolomide for Newly Diagnosed Glioblastoma
- PMID: 26491903
- PMCID: PMC4856584
- DOI: 10.1097/COC.0000000000000136
Clinical, Radiographic, and Pathologic Findings in Patients Undergoing Reoperation Following Radiation Therapy and Temozolomide for Newly Diagnosed Glioblastoma
Abstract
Purpose: Patients with glioblastoma (GBM) frequently deteriorate clinically and radiographically after chemoradiation and may require repeat surgical intervention. We attempted to correlate pathologic findings with preoperative clinical characteristics and survival in patients undergoing reoperation for GBM.
Materials and methods: Patients eligible for this retrospective analysis had pathologically confirmed GBM diagnosed between 2005 and 2010, received standard radiation and temozolomide, and underwent repeat resection within 18 months of diagnosis.
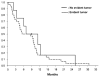
Results: Thirty-eight patients were identified. Median age was 56 years (range, 30 to 80 y), 55% were male, and 66% had baseline performance status ≥90%. Median survival was 16.3 months (95% confidence interval [CI], 13.3-19.8) from initial surgery. At reoperation, 21% of patients had no pathologically evident tumor. Median time from initial diagnosis to second surgery was similar in patients with and without evident tumor (8.5 vs. 8.8 mo, respectively). Patients without evident tumor tended to have a worse performance status. Median overall survival from second surgery was 7 months (95% CI, 4.2-10.1) and 9.1 months (95% CI, 2.1-25.3) for patients with and without evident tumor, respectively. Multivariate proportional hazards analysis showed a hazard ratio for death of 0.61 (95% CI, 0.25-1.49) for patients without evident tumor after adjusting for Karnofsky performance status and second surgical procedure.
Conclusions: GBM patients with and without disease recurrence have similar clinical characteristics at the time of second surgical resection. Pathologic outcomes were not correlated with specific clinical or radiologic characteristics, including the time from diagnosis to reoperation. There was a trend toward improved overall survival among patients without evident tumor at reoperation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Central Brain Tumor Registry of the United States. [Accessed June 7, 2011];CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2007. Available at: http://www.cbtrus.org/2011-NPCR-SEER/WEB-0407-Report-3-3-2011.pdf.
-
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481.
-
- Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41.
-
- Fink J, Born D, Chamberlain MC. Psuedoprogression: relevance with respect to treatment of high-grade gliomas. Curr Treat Options Oncol. 2011;12:240–252. - PubMed
-
- Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9:29–38. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical