Population Genetics and Reproductive Strategies of African Trypanosomes: Revisiting Available Published Data
- PMID: 26491968
- PMCID: PMC4619596
- DOI: 10.1371/journal.pntd.0003985
Population Genetics and Reproductive Strategies of African Trypanosomes: Revisiting Available Published Data
Abstract
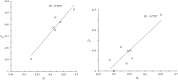
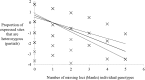
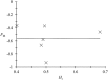
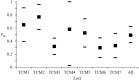
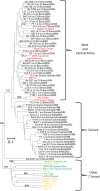
Trypanosomatidae are a dangerous family of Euglenobionta parasites that threaten the health and economy of millions of people around the world. More precisely describing the population biology and reproductive mode of such pests is not only a matter of pure science, but can also be useful for understanding parasite adaptation, as well as how parasitism, specialization (parasite specificity), and complex life cycles evolve over time. Studying this parasite's reproductive strategies and population structure can also contribute key information to the understanding of the epidemiology of associated diseases; it can also provide clues for elaborating control programs and predicting the probability of success for control campaigns (such as vaccines and drug therapies), along with emergence or re-emergence risks. Population genetics tools, if appropriately used, can provide precise and useful information in these investigations. In this paper, we revisit recent data collected during population genetics surveys of different Trypanosoma species in sub-Saharan Africa. Reproductive modes and population structure depend not only on the taxon but also on the geographical location and data quality (absence or presence of DNA amplification failures). We conclude on issues regarding future directions of research, in particular vis-à-vis genotyping and sampling strategies, which are still relevant yet, too often, neglected issues.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Claes F, Buscher P, Touratier L, Goddeeris BM. Trypanosoma equiperdum: master of disguise or historical mistake? Trends Parasitol. 2005;21(7):316–21. - PubMed
-
- MacLeod A, Tweedie A, McLellan S, Taylor S, Cooper A, Sweeney L, et al. Allelic segregation and independent assortment in T. brucei crosses: Proof that the genetic system is Mendelian and involves meiosis. Mol Biochem Parasitol. 2005;143(1):12–9. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

