Schwann cells promote endothelial cell migration
- PMID: 26491999
- PMCID: PMC4955963
- DOI: 10.1080/19336918.2015.1103422
Schwann cells promote endothelial cell migration
Abstract
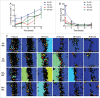
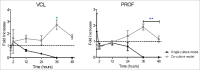
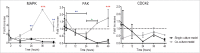
Directed cell migration is a crucial orchestrated process in embryonic development, wound healing, and immune response. The underlying substrate can provide physical and/or chemical cues that promote directed cell migration. Here, using electrospinning we developed substrates of aligned poly(lactic-co-glycolic acid) nanofibres to study the influence of glial cells on endothelial cells (ECs) in a 3-dimensional (3D) co-culture model. ECs build blood vessels and regulate their plasticity in coordination with neurons. Likewise, neurons construct nerves and regulate their circuits in coordination with ECs. In our model, the neuro-vascular cross-talk was assessed using a direct co-culture model of human umbilical vein endothelial cells (HUVECs) and rat Schwann cells (rSCs). The effect of rSCs on ECs behavior was demonstrated by earlier and higher velocity values and genetic expression profiles different of those of HUVECs when seeded alone. We observed 2 different gene expression trends in the co-culture models: (i) a later gene expression of angiogenic factors, such as interleukin-8 (IL-8) and vascular endothelial growth factor (VEGF), and (ii) an higher gene expression of genes involved in actin filaments rearrangement, such as focal adhesion kinase (FAK), Mitogen-activated protein kinase-activated protein kinase 13 (MAPKAPK13), Vinculin (VCL), and Profilin (PROF). These results suggested that the higher ECs migration is mainly due to proteins involved in the actin filaments rearrangement and in the directed cell migration rather than the effect of angiogenic factors. This co-culture model provides an approach to enlighten the neurovascular interactions, with particular focus on endothelial cell migration.
Keywords: cell migration; human umbilical vein endothelial cells; neurovascular; poly(lactic-co-glycolic acid); rat Schwann cells.
Figures




Similar articles
-
[Study on FAK regulation of migration of vascular endothelial cells depending upon focal adhesion proteins].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2013 Jun;30(3):567-71. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2013. PMID: 23865320 Chinese.
-
The effects of biomimetically conjugated VEGF on osteogenesis and angiogenesis of MSCs (human and rat) and HUVECs co-culture models.Colloids Surf B Biointerfaces. 2018 Jul 1;167:550-559. doi: 10.1016/j.colsurfb.2018.04.060. Epub 2018 Apr 30. Colloids Surf B Biointerfaces. 2018. PMID: 29730577
-
Collagen-nanofiber hydrogel composites promote contact guidance of human lymphatic microvascular endothelial cells and directed capillary tube formation.J Biomed Mater Res A. 2013 Jun;101(6):1787-99. doi: 10.1002/jbm.a.34468. Epub 2012 Nov 30. J Biomed Mater Res A. 2013. PMID: 23197422
-
Pivotal micro factors associated with endothelial cells.Chin Med J (Engl). 2019 Aug 20;132(16):1965-1973. doi: 10.1097/CM9.0000000000000358. Chin Med J (Engl). 2019. PMID: 31335473 Free PMC article. Review.
-
The same but different: signaling pathways in control of endothelial cell migration.Curr Opin Cell Biol. 2015 Oct;36:86-92. doi: 10.1016/j.ceb.2015.07.009. Epub 2015 Aug 1. Curr Opin Cell Biol. 2015. PMID: 26241634 Review.
Cited by
-
Potential Role of CD99 Signaling Pathway in Schwann Cell Dysfunction in Diabetic Foot Ulcers Based on Single-Cell Transcriptome Analysis.J Diabetes Res. 2025 May 18;2025:9935400. doi: 10.1155/jdr/9935400. eCollection 2025. J Diabetes Res. 2025. PMID: 40420926 Free PMC article.
-
Functionalizing biomaterials to promote neurovascular regeneration following skeletal muscle injury.Am J Physiol Cell Physiol. 2021 Jun 1;320(6):C1099-C1111. doi: 10.1152/ajpcell.00501.2020. Epub 2021 Apr 14. Am J Physiol Cell Physiol. 2021. PMID: 33852364 Free PMC article. Review.
-
SDF-1α gene-activated collagen scaffold enhances provasculogenic response in a coculture of human endothelial cells with human adipose-derived stromal cells.J Mater Sci Mater Med. 2021 Mar 6;32(3):26. doi: 10.1007/s10856-021-06499-6. J Mater Sci Mater Med. 2021. PMID: 33677751 Free PMC article.
-
Mechanical force promotes the proliferation and extracellular matrix synthesis of human gingival fibroblasts cultured on 3D PLGA scaffolds via TGF‑β expression.Mol Med Rep. 2019 Mar;19(3):2107-2114. doi: 10.3892/mmr.2019.9882. Epub 2019 Jan 21. Mol Med Rep. 2019. PMID: 30664222 Free PMC article.
-
Development of an In Vitro Biomimetic Peripheral Neurovascular Platform.ACS Appl Mater Interfaces. 2022 Jul 20;14(28):31567-31585. doi: 10.1021/acsami.2c03861. Epub 2022 Jul 10. ACS Appl Mater Interfaces. 2022. PMID: 35815638 Free PMC article.
References
-
- Risau W. Mechanisms of angiogenesis. Nature 1997; 386:671-4; PMID:9109485; http://dx.doi.org/10.1038/386671a0 - DOI - PubMed
-
- Shima DT, Mailhos C. Vascular developmental biology: getting nervous. Curr Opin Genet Dev 2000; 10:536-42; PMID:10980432; http://dx.doi.org/10.1016/S0959-437X(00)00124-6 - DOI - PubMed
-
- Park J-A, Choi K-S, Kim S-Y, Kim K-W. Coordinated interaction of the vascular and nervous systems: from molecule- to cell-based approaches. Biochem Biophys Res Commun 2003; 311:247-53; PMID:14592405; http://dx.doi.org/10.1016/j.bbrc.2003.09.129 - DOI - PubMed
-
- Gerber H-P, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular Endothelial Growth Factor Regulates Endothelial Cell Survival through the Phosphatidylinositol 3′-Kinase/Akt Signal Transduction Pathway: REQUIREMENT FOR Flk-1/KDR ACTIVATION. J Biol Chem 1998; 273:30336-43; PMID:9804796; http://dx.doi.org/10.1074/jbc.273.46.30336 - DOI - PubMed
-
- Shigematsu S, Yamauchi K, Nakajima K, Iijima S, Aizawa T, Hashizume K. IGF-1 Regulates Migration and Angiogenesis of Human Endothelial Cells. Endocr J 1999; 46:S59-S62; PMID:12054122; http://dx.doi.org/10.1507/endocrj.46.Suppl_S59 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
