Utilization of an Eilat Virus-Based Chimera for Serological Detection of Chikungunya Infection
- PMID: 26492074
- PMCID: PMC4619601
- DOI: 10.1371/journal.pntd.0004119
Utilization of an Eilat Virus-Based Chimera for Serological Detection of Chikungunya Infection
Abstract
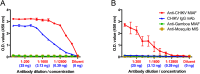
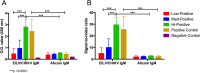
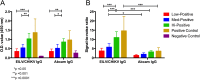
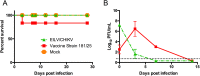
In December of 2013, chikungunya virus (CHIKV), an alphavirus in the family Togaviridae, was introduced to the island of Saint Martin in the Caribbean, resulting in the first autochthonous cases reported in the Americas. As of January 2015, local and imported CHIKV has been reported in 50 American countries with over 1.1 million suspected cases. CHIKV causes a severe arthralgic disease for which there are no approved vaccines or therapeutics. Furthermore, the lack of a commercially available, sensitive, and affordable diagnostic assay limits surveillance and control efforts. To address this issue, we utilized an insect-specific alphavirus, Eilat virus (EILV), to develop a diagnostic antigen that does not require biosafety containment facilities to produce. We demonstrated that EILV/CHIKV replicates to high titers in insect cells and can be applied directly in enzyme-linked immunosorbent assays without inactivation, resulting in highly sensitive detection of recent and past CHIKV infection, and outperforming traditional antigen preparations.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: JHE, FN, and SCW have a patent application entitled "Alphavirus Compositions and Methods of Use” pending, which includes technology reported in this paper. InBios International, Inc. provided support in the form of salaries for authors JN, SM, and SR, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. SR has an ownership at InBios. InBios has a license to the pending patent. This does not alter our adherence to all PLOS policies on sharing data and materials.
Figures


Similar articles
-
Development and evaluation of baculovirus-expressed Chikungunya virus E1 envelope proteins for serodiagnosis of Chikungunya infection.J Virol Methods. 2014 Sep;206:67-75. doi: 10.1016/j.jviromet.2014.05.014. Epub 2014 May 29. J Virol Methods. 2014. PMID: 24880071
-
A chikungunya fever vaccine utilizing an insect-specific virus platform.Nat Med. 2017 Feb;23(2):192-199. doi: 10.1038/nm.4253. Epub 2016 Dec 19. Nat Med. 2017. PMID: 27991917 Free PMC article.
-
Enzyme-linked immunosorbent assay using recombinant envelope protein 2 antigen for diagnosis of Chikungunya virus.Virol J. 2018 Jul 24;15(1):112. doi: 10.1186/s12985-018-1028-1. Virol J. 2018. PMID: 30041676 Free PMC article.
-
Diagnostic accuracy of serological tests for the diagnosis of Chikungunya virus infection: A systematic review and meta-analysis.PLoS Negl Trop Dis. 2022 Feb 4;16(2):e0010152. doi: 10.1371/journal.pntd.0010152. eCollection 2022 Feb. PLoS Negl Trop Dis. 2022. PMID: 35120141 Free PMC article.
-
Beyond Fever and Pain: Diagnostic Methods for Chikungunya Virus.J Clin Microbiol. 2019 May 24;57(6):e00350-19. doi: 10.1128/JCM.00350-19. Print 2019 Jun. J Clin Microbiol. 2019. PMID: 30995993 Free PMC article. Review.
Cited by
-
Perspectives on New Vaccines against Arboviruses Using Insect-Specific Viruses as Platforms.Vaccines (Basel). 2021 Mar 16;9(3):263. doi: 10.3390/vaccines9030263. Vaccines (Basel). 2021. PMID: 33809576 Free PMC article. Review.
-
Risk factors for infection with chikungunya and Zika viruses in southern Puerto Rico: A community-based cross-sectional seroprevalence survey.PLoS Negl Trop Dis. 2022 Jun 13;16(6):e0010416. doi: 10.1371/journal.pntd.0010416. eCollection 2022 Jun. PLoS Negl Trop Dis. 2022. PMID: 35696355 Free PMC article.
-
Commensal Viruses of Mosquitoes: Host Restriction, Transmission, and Interaction with Arboviral Pathogens.Evol Bioinform Online. 2017 Jan 10;12(Suppl 2):35-44. doi: 10.4137/EBO.S40740. eCollection 2016. Evol Bioinform Online. 2017. PMID: 28096646 Free PMC article. Review.
-
Molecular and Clinical Characterization of Chikungunya Virus Infections in Southeast Mexico.Viruses. 2018 May 9;10(5):248. doi: 10.3390/v10050248. Viruses. 2018. PMID: 29747416 Free PMC article.
-
Development of Vaccines for Chikungunya Fever.J Infect Dis. 2016 Dec 15;214(suppl 5):S488-S496. doi: 10.1093/infdis/jiw271. J Infect Dis. 2016. PMID: 27920179 Free PMC article. Review.
References
-
- Kuhn RJ. Togaviridae: The viruses and their replication In: Straus SE, Roizman B, Martin MA, Lamb RA, Griffin DE, Howley PM, Knipe DM, editors. Fields Virology. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. p. 1001–22.
-
- Weston JH, Welsh MD, McLoughlin MF, Todd D. Salmon pancreas disease virus, an alphavirus infecting farmed atlantic salmon, salmo salar L. Virology 1999, April 10;256(2):188–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

